Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease - PubMed (original) (raw)
Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease
Miranda L Bader Lange et al. Neurobiol Dis. 2008 Mar.
Abstract
Oxidative stress, a hallmark of Alzheimer disease (AD), has been shown to induce lipid peroxidation and apoptosis disrupting cellular homeostasis. Normally, the aminophospholipid phosphatidylserine (PtdSer) is asymmetrically distributed on the cytosolic leaflet of the lipid bilayer. Under oxidative stress conditions, asymmetry is altered, characterized by the appearance of PtdSer on the outer leaflet, to initiate the first stages of an apoptotic process. PtdSer asymmetry is actively maintained by the ATP-dependent translocase flippase, whose function is inhibited if covalently bound by lipid peroxidation products, 4-hydroxynonenal (HNE) and acrolein, within the membrane bilayer in which they are produced. Additionally, pro-apoptotic proteins Bax and caspase-3 have been implemented in the oxidative modification of PtdSer resulting in subsequent asymmetric collapse, while anti-apoptotic protein Bcl-2 has been found to prevent this process. The current investigation focused on detection of PtdSer on the outer leaflet of the bilayer in synaptosomes from brain of subjects with AD and amnestic mild cognitive impairment (MCI), as well as expression levels of apoptosis-related proteins Bcl-2, Bax, and caspase-3. Fluorescence and Western blot analysis suggest PtdSer exposure on the outer leaflet is significantly increased in brain from subjects with MCI and AD contributing to early apoptotic elevation of pro- and anti-apoptotic proteins and finally neuronal loss. MCI is considered a possible transition point between normal cognitive aging and probable AD. Brain from subjects with MCI is reported to have increased levels of tissue oxidation; therefore, the results of this study could mark the progression of patients with MCI into AD. This study contributes to a model of apoptosis-specific oxidation of phospholipids consistent with the notion that PtdSer exposure is required for apoptotic-cell death.
Figures
Figure 1. NBD-PS assay in synaptosomes from brain of subjects with MCI and AD
HumanMCI, AD, and control synaptosomes were treated with the fluorescent phospholipid NBD-PS. PtdSer exposure to the outer leaflet of the membrane bilayer is measured as the percent decrease in fluorescence signal after Na2S2O4 quenching. The control value was set to 100%, to which experimental values were compared. These data, in arbitrary units on the ordinate axis, are presented as mean ±S.D. AD, N=5, *P<0.0001; MCI, N=5, **P<0.00001.
Figure 2. PMI NBD-PS assay
FVB mouse synaptosomes were treated with the fluorescent phospholipid NBD-PS. PtdSer exposure to the outer leaflet of the plasma membrane is measured as a decrease in fluorescence signal, compared to 0 h, after quenching with Na2S2O4. Since samples were not compared as a percentage of control, negative values are not seen, but graphical interpretation is relatively the same. A fluorescence decrease, compared to 0 h, denotes an increased amount of PtdSer exposed to the lipid bilayer outer leaflet. Data shown in arbitrary fluorescence units on the ordinate axis as mean ±S.D. 0 h, N=3; 2.8 h, N=2, P<0.65; 3.4 h, N=3, P<0.80.
Figure 3. Mg2+ATPase activity assay
FVB mouse synaptosome Mg2+ATPase activity was measured to represent flippase activity, an ATP-dependent membrane bound translocase. Decreased UV-absorbance (810 nm) compared to 0 h denotes decreased Mg2+ATPase activity. Data shown, in arbitrary absorbance units, as mean ±S.D. 0 h, N=3; 2.8 h, N=2, *P<0.04; 3.5 h, N=3, P<0.26.
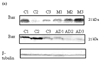
Figure 4. Bcl-2 levels in brain from subjects with MCI and AD
Bcl-2 levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of Bcl-2 protein present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.006; in (b) AD N=3, *P<0.04.
Figure 4. Bcl-2 levels in brain from subjects with MCI and AD
Bcl-2 levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of Bcl-2 protein present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.006; in (b) AD N=3, *P<0.04.
Figure 5. Bax levels in brain from subjects with MCI and AD
Bax levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of Bax protein present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.03; in (b) AD N=3, *P<0.005.
Figure 5. Bax levels in brain from subjects with MCI and AD
Bax levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of Bax protein present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.03; in (b) AD N=3, *P<0.005.
Figure 6. Caspase-3 levles in brain from subjects with MCI and AD
Caspase-3 levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of caspase-3 present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.02; in (b) AD N=3, *P<0.04.
Figure 6. Caspase-3 levles in brain from subjects with MCI and AD
Caspase-3 levels were measured in IPL of MCI and AD patients by Western blot analysis. (a) MCI and AD gels were run with equal amounts of protein (75 µg/lane). Increased band intensity after normalization with β-tubulin represents an increase in the amount of caspase-3 present. (b) & (c) Graphical analysis of MCI and AD band intensities, respectively. The control value was set to 100%, to which experimental values were compared. Data shown in arbitrary units on the ordinate axis as mean ±S.D. in (a) MCI, N=3, *P<0.02; in (b) AD N=3, *P<0.04.
Similar articles
- Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer's disease.
Cenini G, Sultana R, Memo M, Butterfield DA. Cenini G, et al. J Cell Mol Med. 2008 Jun;12(3):987-94. doi: 10.1111/j.1582-4934.2008.00163.x. J Cell Mol Med. 2008. PMID: 18494939 Free PMC article. - Age-related loss of phospholipid asymmetry in APP(NLh)/APP(NLh) x PS-1(P264L)/PS-1(P264L) human double mutant knock-in mice: relevance to Alzheimer disease.
Bader Lange ML, St Clair D, Markesbery WR, Studzinski CM, Murphy MP, Butterfield DA. Bader Lange ML, et al. Neurobiol Dis. 2010 Apr;38(1):104-15. doi: 10.1016/j.nbd.2010.01.004. Epub 2010 Jan 18. Neurobiol Dis. 2010. PMID: 20083199 Free PMC article. - Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: implications for Alzheimer's disease.
Castegna A, Lauderback CM, Mohmmad-Abdul H, Butterfield DA. Castegna A, et al. Brain Res. 2004 Apr 9;1004(1-2):193-7. doi: 10.1016/j.brainres.2004.01.036. Brain Res. 2004. PMID: 15033435 - Role of by-products of lipid oxidation in Alzheimer's disease brain: a focus on acrolein.
Singh M, Dang TN, Arseneault M, Ramassamy C. Singh M, et al. J Alzheimers Dis. 2010;21(3):741-56. doi: 10.3233/JAD-2010-100405. J Alzheimers Dis. 2010. PMID: 20634576 Review.
Cited by
- Specific Phospholipid Modulation by Muscarinic Signaling in a Rat Lesion Model of Alzheimer's Disease.
Llorente-Ovejero A, Martínez-Gardeazabal J, Moreno-Rodríguez M, Lombardero L, González de San Román E, Manuel I, Giralt MT, Rodríguez-Puertas R. Llorente-Ovejero A, et al. ACS Chem Neurosci. 2021 Jun 16;12(12):2167-2181. doi: 10.1021/acschemneuro.1c00169. Epub 2021 May 26. ACS Chem Neurosci. 2021. PMID: 34037379 Free PMC article. - Oxidative stress and β-amyloid protein in Alzheimer's disease.
Cai Z, Zhao B, Ratka A. Cai Z, et al. Neuromolecular Med. 2011 Dec;13(4):223-50. doi: 10.1007/s12017-011-8155-9. Epub 2011 Sep 8. Neuromolecular Med. 2011. PMID: 21901428 Review. - Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis.
Sultana R, Perluigi M, Butterfield DA. Sultana R, et al. Acta Neuropathol. 2009 Jul;118(1):131-50. doi: 10.1007/s00401-009-0517-0. Epub 2009 Mar 14. Acta Neuropathol. 2009. PMID: 19288120 Free PMC article. Review. - Antioxidants in central nervous system diseases: preclinical promise and translational challenges.
Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Kamat CD, et al. J Alzheimers Dis. 2008 Nov;15(3):473-93. doi: 10.3233/jad-2008-15314. J Alzheimers Dis. 2008. PMID: 18997301 Free PMC article. Review.
References
- Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu. Rev. Physiol. 2003;65:701–734. - PubMed
- Bitbol M, Fellmann P, Zachowski A, Devaux PF. Ion regulation of phosphatidylserine and phosphatidylethanolamine outside-inside translocation in human erythrocytes. Biochim. Biophys. Acta. 1987;904:268–282. - PubMed
- Bruce-Keller AJ, Begley JG, Fu W, Butterfield DA, Bredesen DE, Hutchins JB, Hensley K, Mattson MP. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem. 1998;70:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010836-130011/AG/NIA NIH HHS/United States
- P01 AG005119-110001/AG/NIA NIH HHS/United States
- P01 AG010836/AG/NIA NIH HHS/United States
- AG-10836/AG/NIA NIH HHS/United States
- P01 AG005119/AG/NIA NIH HHS/United States
- AG-05119/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials