Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus - PubMed (original) (raw)
Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus
Joshua J Breunig et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The lifelong addition of neurons to the hippocampus is a remarkable form of structural plasticity, yet the molecular controls over proliferation, neuronal fate determination, survival, and maturation are poorly understood. Expression of Notch1 was found to change dynamically depending on the differentiation state of neural precursor cells. Through the use of inducible gain- and loss-of-function of Notch1 mice we show that this membrane receptor is essential to these distinct processes. We found in vivo that activated Notch1 overexpression induces proliferation, whereas gamma-secretase inhibition or genetic ablation of Notch1 promotes cell cycle exit, indicating that the level of activated Notch1 regulates the magnitude of neurogenesis from postnatal progenitor cells. Abrogation of Notch signaling in vivo or in vitro leads to a transition from neural stem or precursor cells to transit-amplifying cells or neurons. Further, genetic Notch1 manipulation modulates survival and dendritic morphology of newborn granule cells. These results provide evidence for the expansive prevalence of Notch signaling in hippocampal morphogenesis and plasticity, suggesting that Notch1 could be a target of diverse traumatic and environmental modulators of adult neurogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
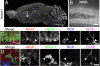
Notch1 and Ascl1 expression in the postnatal hippocampus. (A) In situ hybridization for Notch1 mRNA. (B) Low-magnification confocal image of the immunohistochemical localization of Notch1 in P24 hippocampal coronal sections. Immunoreactivity is present in virtually all mature neurons and astrocytes. (C) Confocal image of a section stained for NICD (red), NeuN (green), Dcx (blue), and Gfap (magenta) taken through the dentate gyrus. There is prominent colocalization of NeuN and NICD in mature neurons as well as significant localization of NICD in the cytoplasm of Gfap+ astrocytes (filled arrowhead) and an absence of NICD in the nearby Dcx+ cell (empty arrowhead). (D) Immunostaining for NICD (red), Ascl1 (green), Dcx (blue), and Gfap (magenta). Ascl1+ doublet with one Ascl1+ nucleus colocalizing with Gfap (filled arrowhead) and the other Gfap- (empty arrowhead). NICD did not colocalize with the mostly nuclear Ascl1 protein in either case. GCL, granule cell layer; SGZ, subgranular zone. (Scale bars: B, 100 μm; C and D, 10 μm.)
Fig. 2.
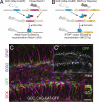
Cre-mediated manipulation of Notch1. (A and B) Schematic of mouse breedings and ligand-induced Cre recombination. (A) GCE mice are crossed with loxP-flanked (“floxed”) Notch1 mice. GCE; Notch1fl/fl mice are given tamoxifen, causing the Cre-ERT2 fusion protein—which is otherwise bound to heat shock protein 90 in the cytoplasm and thus is inactive—to translocate to the nucleus where it recombines paired loxP sites, ablating the Notch1 protein. (B) GCE mice are crossed with NICD transgenic mice. GCE; NICD mice are given tamoxifen, causing nuclear translocation of the CreER protein which recombines the loxP sites, excising the “STOP” codon and inducing NICD protein transcription and translation. (C) Three doses of Tamoxifen induce recombination in a significant population of SGZ cells as determined by reporter expression (GFP, green). (Scale bar: C, 50 μm.)
Fig. 3.
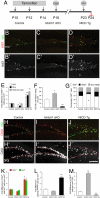
Genetic manipulation of Notch influences cell proliferation, and cell cycle exit. (A) Schematic of the injection paradigm for tamoxifen, CldU, and IdU. (B–D) Ki67 (red)/IdU (green) immunostaining of the dentate gyrus of control (Ctrl), NICD overexpressing (NICD Tg), and Notch1 ablated (Notch1 cKO) animals. (B′–D′) Ki67 signal from B–D shown with enhanced contrast. (E) NICD overexpression drastically increases the number of proliferating cells in the subgranular zone (SGZ), hilus, and molecular layer when compared with controls and Notch1 cKO groups. The average number of Ki67 positive cells per mm3 in control animals was used for normalization. (F) Opposite effects on cell cycle exit are seen when comparing NICD Tg and Notch1 cKO animals with controls. Significantly more cells leave the cell cycle in Notch1 cKO animals than in controls or NICD Tg animals where only 7% of cells leave the cell cycle. (G) The phenotype of proliferating cells is skewed in Notch1 cKO animals where fewer Gfap+ cells proliferate at a reduced level. (Immunostaining for Dcx/Gfap is not shown for the sake of clarity.) (H–J) CldU (red)/IdU (green) immunohistochemistry on DG tissue sections. (H′-J′) CldU signal from H–J is shown with enhanced contrast and the upper limit of the SGZ is labeled with a dotted red line. (K) The number of cells labeled by CldU and IdU increase in the NICD Tg group. The average number of CldU or IdU positive cells per mm3 in control animals was used for normalization. (L) CldU/IdU double-positive cell number increases almost threefold in NICD Tg mice over control and Notch1 cKO animals. The average number of CldU/IdU double-positive cells per mm3 in control animals was used for normalization. (M) CldU+ cells, which synthesized DNA one week before perfusion, remain preferentially in the subgranular zone in NICD Tg animals whereas in Notch1 cKO animals CldU+ cells are preferentially found in the granule cell layer. The average number of CldU-positive cells per mm3 in control animals was used for normalization. Note n = 6 for each experimental group. Asterisks indicate a statistical difference between experimental groups (*, P < 0.05; **, P < 0.001; Student's t test: E–G, K–M). Error bars represent SEM. (Scale bars: B–D′ and H–J′, 100 μm.)
Fig. 4.
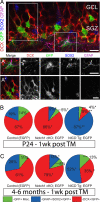
Cell autonomous changes in cell fate in the DG. (A) Induced GFP (green) reporter expression in the SGZ in tissue immunostained for Dcx (red), Sox2 (blue), and Gfap (magenta). Example of a GFP+ glial cell (A′) showing expression of Sox2 and Gfap. (A″) GFP+/Dcx+ neurons in the GCL. (B) Notch1 cKO animals show a preferential generation of neurons, but NICD Tg mice display a dramatic maintenance of glial cells at the expense of neurons. (C) This same reciprocal change in cell fates is seen in 4- to 6-month-old mice given tamoxifen 1 week before killing. Note n = 4 for each experimental group. Asterisks indicate a statistical difference between experimental groups (*, P < 0.05; **, P < 0.001; Student's t test; B and E). Error bars represent SEM. (Scale bars: A-A″, C-C′, 10 μm; D, 50 μm.)
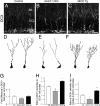
Fig. 5.
Notch-signaling modulates the dendritic arborization of maturing hippocampal neurons. (A–C) Confocal image of Dcx immunohistochemistry in the dentate gyrus at P37. Representative examples of Dcx+/GFP+ neurons in D Control, (E) Notch1 cKO, and (F) NICD Tg mice. GFP will label only newly born cells. Representative examples of the more ramified cells in each group are show. (G) Dcx+ cell numbers are not significantly different in the SGZ of NICD Tg and Notch1 cKO animals when compared with controls. The average number of Dcx+ cells per mm3 in control animals was used for normalization. (H) NICD Tg animals display more dendrites per Dcx+ cell body. Notch1 cKO animals show a significant drop in dendritic complexity. (I) NICD Tg animals have more varicosities per Dcx+ cell whereas Notch1 cKO mice show a significant decrease. Note n = 6 for each experimental group. Asterisks indicate a statistical difference between experimental groups (*, P < 0.05; **, P < 0.001; Student's t test; G–I). Error bars represent SEM. (Scale bars: A–C, 30 μm.)
Similar articles
- Notch1 deficiency in postnatal neural progenitor cells in the dentate gyrus leads to emotional and cognitive impairment.
Feng S, Shi T, Qiu J, Yang H, Wu Y, Zhou W, Wang W, Wu H. Feng S, et al. FASEB J. 2017 Oct;31(10):4347-4358. doi: 10.1096/fj.201700216RR. Epub 2017 Jun 13. FASEB J. 2017. PMID: 28611114 - Reelin and Notch1 cooperate in the development of the dentate gyrus.
Sibbe M, Förster E, Basak O, Taylor V, Frotscher M. Sibbe M, et al. J Neurosci. 2009 Jul 1;29(26):8578-85. doi: 10.1523/JNEUROSCI.0958-09.2009. J Neurosci. 2009. PMID: 19571148 Free PMC article. - Postnatal dysregulation of Notch signal disrupts dendrite development of adult-born neurons in the hippocampus and contributes to memory impairment.
Ding XF, Gao X, Ding XC, Fan M, Chen J. Ding XF, et al. Sci Rep. 2016 May 13;6:25780. doi: 10.1038/srep25780. Sci Rep. 2016. PMID: 27173138 Free PMC article. - Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus.
Rahimi O, Claiborne BJ. Rahimi O, et al. Prog Brain Res. 2007;163:167-81. doi: 10.1016/S0079-6123(07)63010-6. Prog Brain Res. 2007. PMID: 17765718 Review. - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
Cited by
- Neuroprotective Properties of Asiatic Acid against 5-Fluorouracil Chemotherapy in the Hippocampus in an Adult Rat Model.
Welbat JU, Chaisawang P, Pannangrong W, Wigmore P. Welbat JU, et al. Nutrients. 2018 Aug 9;10(8):1053. doi: 10.3390/nu10081053. Nutrients. 2018. PMID: 30096914 Free PMC article. - Adult Neurogenesis in Epileptogenesis: An Update for Preclinical Finding and Potential Clinical Translation.
Chen L, Wang Y, Chen Z. Chen L, et al. Curr Neuropharmacol. 2020;18(6):464-484. doi: 10.2174/1570159X17666191118142314. Curr Neuropharmacol. 2020. PMID: 31744451 Free PMC article. Review. - Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis.
Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Lavado A, et al. PLoS Biol. 2010 Aug 17;8(8):e1000460. doi: 10.1371/journal.pbio.1000460. PLoS Biol. 2010. PMID: 20808958 Free PMC article. - Waking up quiescent neural stem cells: Molecular mechanisms and implications in neurodevelopmental disorders.
Ding WY, Huang J, Wang H. Ding WY, et al. PLoS Genet. 2020 Apr 23;16(4):e1008653. doi: 10.1371/journal.pgen.1008653. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32324743 Free PMC article. Review. - Differential expression of Notch family members in astrocytomas and medulloblastomas.
Xu P, Yu S, Jiang R, Kang C, Wang G, Jiang H, Pu P. Xu P, et al. Pathol Oncol Res. 2009 Dec;15(4):703-10. doi: 10.1007/s12253-009-9173-x. Epub 2009 May 8. Pathol Oncol Res. 2009. PMID: 19424825
References
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. - PubMed
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. - PubMed
- Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. - PubMed
- Lai K, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD045481/HD/NICHD NIH HHS/United States
- AG019394/AG/NIA NIH HHS/United States
- R01 NS047200/NS/NINDS NIH HHS/United States
- HD045481/HD/NICHD NIH HHS/United States
- R01 MH067715/MH/NIMH NIH HHS/United States
- R01 AG019394/AG/NIA NIH HHS/United States
- NS047200/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases