Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia - PubMed (original) (raw)
Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia
Nellie Kalcheva et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Gap junction channels are required for normal cardiac impulse propagation, and gap junction remodeling is associated with enhanced arrhythmic risk. Oculodentodigital dysplasia (ODDD) is a multisystem syndrome due to mutations in the connexin43 (Cx43) gap junction channel gene. To determine the effects of a human connexin channelopathy on cardiac electrophysiology and arrhythmogenesis, we generated a murine model of ODDD by introducing the disease-causing I130T mutant allele into the mouse genome. Cx43 abundance was markedly reduced in mutant hearts with preferential loss of phosphorylated forms that interfered with trafficking and assembly of gap junctions in the junctional membrane. Dual whole-cell patch-clamp studies showed significantly lower junctional conductance between neonatal cell pairs from mutant hearts, and optical mapping of isolated-perfused hearts with voltage-sensitive dyes demonstrated significant slowing of conduction velocity. Programmed electrical stimulation revealed a markedly increased susceptibility to spontaneous and inducible ventricular tachyarrhythmias. In summary, our data demonstrate that the I130T mutation interferes with Cx43 posttranslational processing, resulting in diminished cell-cell coupling, slowing of impulse propagation, and a proarrhythmic substrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
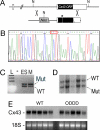
Generation of Cx43I130T/+ Mutant mice. (A) The Cx43 locus (Upper) and targeting vector (Lower) are shown. The locus comprises two exons with the ORF within exon 2. The neomycin (Neo) resistance cassette, loxP sites (triangles), and location of the I130T mutation (asterisk) within the targeting vector are shown. NcoI restriction sites (N) are indicated. (B) Sequence analysis of targeting vector demonstrating introduction of the I130T missense mutation (ACT codon in red box). (C) PCR assay with primer pairs that flank the loxP site residing in the 3′ untranslated region of the targeted allele. Lanes include ladder (L), no input DNA (−), DNA from heterozygous targeted ES cells (ES), or DNA from an F1 mutant mouse (M). PCR products indicative of both the wild-type (WT) and mutant (Mut) I130T alleles are present. (D) Southern blot analysis of NcoI-digested genomic DNA from F2 offspring positive for heterozygosity by PCR assay, showing the expected polymorphism and confirming transmission of both the wild-type and mutant alleles. (E) Northern blot analysis of wild-type (WT) and ODDD mutant hearts demonstrate comparable expression of Cx43. Ethidium bromide staining of 18S ribosomal RNA is also shown.
Fig. 2.
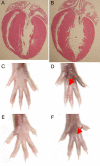
Cardiac and limb morphology in ODDD mutant mice. (A and B) Four-chamber view of 3-month-old wild-type (A) and ODDD mutant (B) hearts demonstrates preserved cardiac morphology. (C–E) Comparison of left (C and D) and right (E and F) hind limbs from wild-type and heterozygous ODDD mutant mice. Syndactyly in mutants is indicated by red arrows.
Fig. 3.
Aberrant expression of Cx43 in ODDD mutant hearts. (A) Western blot analysis of wild-type (WT) and ODDD mutant (ODDD) hearts using rabbit antibody 18B, which reacts with all forms of Cx43 (panCx43) and mouse monoclonal CX1B1, which preferentially reacts with the P0 form of Cx43 (np-Cx43). Lanes 1–4 are from wild-type hearts, and lanes 5–8 are from ODDD hearts. Signals were visualized with enhanced chemiluminescence. (B) Western blot analysis using mouse monoclonal Cx43NT1, which recognizes all forms of Cx43, and rabbit anti-pS325/328/330-Cx43 and rabbit anti-pS365-Cx43 antibodies, which recognize specific phosphorylated forms of Cx43. Lanes 1, 3, 5, 7, and 9 are from wild-type hearts, and lanes 2, 4, 6, and 8 are from ODDD hearts. Equivalency of loading was verified by probing for vinculin (vinc) and GAPDH. Signals were visualized and quantified using the Li-Cor imaging system. Arrows in A and B indicate P0, P1, and P2 forms of Cx43. (C) Abundance of total Cx43, P0, and P1/P2 forms of Cx43 in wild-type (■) and ODDD (□) hearts. Values are expressed as mean ± SEM relative to total Cx43 levels in wild-type hearts. (D) Western blot analysis for zona occludens-1 (ZO-1), pan-cadherin (panCad), plakoglobin (plako), β-catenin (β-Cat), desmoplakin (desmo), and GAPDH. Lanes 1–4 are from wild-type hearts, and lanes 5–8 are from ODDD hearts.
Fig. 4.
Cx43 gap junctions are diminished in ODDD mutant hearts. Immunohistochemical staining of wild-type (A, C, and E) and ODDD mutant hearts (B, D, and F) with antibodies recognizing all forms of Cx43 (A and B), phosphoS325/328/330-Cx43 (C and D), and phosphoS365-Cx43 (E and F). Phosphorylated forms of Cx43 are virtually absent in ODDD mutant hearts. (Scale bar: 20 μm.)
Fig. 5.
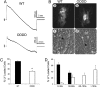
Coupling is reduced in ODDD mutant myocytes. (A) Junctional currents recorded between pairs of cardiac myocytes isolated from wild-type (Upper) and ODDD heterozygotes (Lower). Both cells were voltage-clamped with patch-type electrodes and held at 0 mV; junctional current (_I_j) was recorded in one cell when the other's voltage was ramped from −100 to 100 mV at 27 mV/sec. Note nonlinearities in _I_j at large transjunctional voltages for both genotypes, due to voltage-dependent gating of junctional channels. (B) Representative examples of Lucifer yellow dye coupling in cultures of wild-type (a and c) and heterozygous ODDD mutant (b and d) myocytes. (C) Quantification of dye transfer assay shows significantly reduced coupling in ODDD cultures compared with wild-type (WT) cultures. (D) The rate of dye transfer was significantly slower between ODDD mutant myocytes (ρ) compared with wild type (ν).
Fig. 6.
Abnormal conduction and arrhythmias in ODDD mutant mice. (A) Representative signal-averaged surface electrocardiograms (lead II) from a wild-type (WT) and an ODDD mutant mouse. Note the diminished QRS amplitude in the mutant. (B) Optical mapping of the left ventricular surface of a representative wild-type heart and three individual ODDD mutant hearts. The temporal and spatial scales are indicated and are identical for all images. Conduction velocity, determined from pixels between 1 and 3 mm from the site of stimulation, is significantly slowed in the ODDD hearts, as indicated by closer spacing of isochrones (1 ms apart). (C) Programmed electrical stimulation showing examples of return of sinus rhythm in a wild-type heart (Left), induction of sustained VT in an ODDD heart (Center), and spontaneous VT in an ODDD heart (Right). Tracings each include 2 sec of recordings.
Similar articles
- The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans.
Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. Dobrowolski R, et al. Hum Mol Genet. 2008 Feb 15;17(4):539-54. doi: 10.1093/hmg/ddm329. Epub 2007 Nov 13. Hum Mol Genet. 2008. PMID: 18003637 Free PMC article. - Modulation of cardiac gap junction expression and arrhythmic susceptibility.
Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Danik SB, et al. Circ Res. 2004 Nov 12;95(10):1035-41. doi: 10.1161/01.RES.0000148664.33695.2a. Epub 2004 Oct 21. Circ Res. 2004. PMID: 15499029 Free PMC article. - Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia.
Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Shibayama J, et al. Circ Res. 2005 May 27;96(10):e83-91. doi: 10.1161/01.RES.0000168369.79972.d2. Epub 2005 May 5. Circ Res. 2005. PMID: 15879313 - Cardiac conduction in isolated hearts of genetically modified mice--Connexin43 and salts.
George SA, Poelzing S. George SA, et al. Prog Biophys Mol Biol. 2016 Jan;120(1-3):189-98. doi: 10.1016/j.pbiomolbio.2015.11.004. Epub 2015 Nov 25. Prog Biophys Mol Biol. 2016. PMID: 26627143 Free PMC article. Review. - Intracellular trafficking pathways of Cx43 gap junction channels.
Epifantseva I, Shaw RM. Epifantseva I, et al. Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):40-47. doi: 10.1016/j.bbamem.2017.05.018. Epub 2017 May 30. Biochim Biophys Acta Biomembr. 2018. PMID: 28576298 Free PMC article. Review.
Cited by
- Cardiac dynamics: Alternans and arrhythmogenesis.
Tse G, Wong ST, Tse V, Lee YT, Lin HY, Yeo JM. Tse G, et al. J Arrhythm. 2016 Oct;32(5):411-417. doi: 10.1016/j.joa.2016.02.009. Epub 2016 Mar 28. J Arrhythm. 2016. PMID: 27761166 Free PMC article. Review. - Revisiting K(+) channel-dependent electrical remodeling in the border zone.
Curran J, Mohler PJ. Curran J, et al. J Cardiovasc Electrophysiol. 2013 Oct;24(10):1154-6. doi: 10.1111/jce.12189. Epub 2013 Jun 17. J Cardiovasc Electrophysiol. 2013. PMID: 23773490 Free PMC article. No abstract available. - A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia.
Smyth JW, Zhang SS, Sanchez JM, Lamouille S, Vogan JM, Hesketh GG, Hong T, Tomaselli GF, Shaw RM. Smyth JW, et al. Traffic. 2014 Jun;15(6):684-99. doi: 10.1111/tra.12169. Epub 2014 Apr 9. Traffic. 2014. PMID: 24612377 Free PMC article. - Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium.
Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Smyth JW, et al. J Clin Invest. 2010 Jan;120(1):266-79. doi: 10.1172/JCI39740. Epub 2009 Dec 28. J Clin Invest. 2010. PMID: 20038810 Free PMC article. - Connexins and Disease.
Delmar M, Laird DW, Naus CC, Nielsen MS, Verselis VK, White TW. Delmar M, et al. Cold Spring Harb Perspect Biol. 2018 Sep 4;10(9):a029348. doi: 10.1101/cshperspect.a029348. Cold Spring Harb Perspect Biol. 2018. PMID: 28778872 Free PMC article. Review.
References
- Saffitz JE, Schuessler RB, Yamada KA. Cardiovasc Res. 1999;42:309–317. - PubMed
- Seki A, Coombs W, Taffet SM, Delmar M. Heart Rhythm. 2004;1:227–233. - PubMed
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Circ Res. 2005;96:e83–e91. - PubMed
- Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder GM, Laird DW. J Biol Chem. 2005;280:11458–11466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM55632/GM/NIGMS NIH HHS/United States
- R01 GM055632/GM/NIGMS NIH HHS/United States
- R01 GM055632-09/GM/NIGMS NIH HHS/United States
- HL82727/HL/NHLBI NIH HHS/United States
- R01 HL082727-02/HL/NHLBI NIH HHS/United States
- R01 HL082727/HL/NHLBI NIH HHS/United States
- R01 HL064757-08/HL/NHLBI NIH HHS/United States
- HL64757/HL/NHLBI NIH HHS/United States
- R01 HL064757/HL/NHLBI NIH HHS/United States
- P01 HD032573/HD/NICHD NIH HHS/United States
- R01 GM055632-10/GM/NIGMS NIH HHS/United States
- HD32573/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous