Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions - PubMed (original) (raw)
. 2008 Jan;135(2):367-75.
doi: 10.1242/dev.013540. Epub 2007 Dec 12.
He Huang, Keiko Tamai, Xinjun Zhang, Yuko Harada, Chika Yokota, Karla Almeida, Jianbo Wang, Brad Doble, Jim Woodgett, Anthony Wynshaw-Boris, Jen-Chieh Hsieh, Xi He
Affiliations
- PMID: 18077588
- PMCID: PMC5328672
- DOI: 10.1242/dev.013540
Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions
Xin Zeng et al. Development. 2008 Jan.
Abstract
Canonical Wnt/beta-catenin signaling has central roles in development and diseases, and is initiated by the action of the frizzled (Fz) receptor, its coreceptor LDL receptor-related protein 6 (Lrp6), and the cytoplasmic dishevelled (Dvl) protein. The functional relationships among Fz, Lrp6 and Dvl have long been enigmatic. We demonstrated previously that Wnt-induced Lrp6 phosphorylation via glycogen synthase kinase 3 (Gsk3) initiates Wnt/beta-catenin signaling. Here we show that both Fz and Dvl functions are critical for Wnt-induced Lrp6 phosphorylation through Fz-Lrp6 interaction. We also show that axin, a key scaffolding protein in the Wnt pathway, is required for Lrp6 phosphorylation via its ability to recruit Gsk3, and inhibition of Gsk3 at the plasma membrane blocks Wnt/beta-catenin signaling. Our results suggest a model that upon Wnt-induced Fz-Lrp6 complex formation, Fz recruitment of Dvl in turn recruits the axin-Gsk3 complex, thereby promoting Lrp6 phosphorylation to initiate beta-catenin signaling. We discuss the dual roles of the axin-Gsk3 complex and signal amplification by Lrp6-axin interaction during Wnt/beta-catenin signaling.
Figures
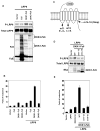
Fig. 1. Fz function is required for Wnt-induced LRP6 phosphorylation
(A) Shisa inhibited Wnt3a-induced LRP6 phosphorylation. HEK293T cells co-transfected with LRP6 plus Flag-tagged Shisa (or a control vector) were incubated with Wnt3a conditioned medium (CM) or the control CM for 1 hour. Shisa inhibited Wnt3a-induced β-catenin accumulation in the cytosol. β-actin: a loading control. (B) L cells stably transfected with shRNAs against Fz2 and Fz7 [L (Fz-) cell] showed diminished Wnt-induced LRP6 (endogenous) phosphorylation. The L (Fz-) cells were treated for 1hour with increasing concentrations of Wnt3a CM. These L (Fz-) cells also exhibited attenuated β-catenin stabilization in response to Wnt3a compared to the control L cells. (C) Human Fz5 expression rescued Wnt3a-induced LRP6 (endogenous) phosphorylation in the L (Fz-) cells. The stable clones of L (Fz-) cell transfected with Fz5 (or the control vector) were pooled together and assayed.
Fig. 2. Forced Fz-LRP6 association activates LRP6 phosphorylation
(A, B) DKK1-Fz5, but neither Fz5 nor DKK3-Fz5, activated LRP6 phosphorylation (A) and TCF/β-catenin reporter expression (B) in HEK293T cells. The expression level of DKK1-Fz5 and DKK3-Fz5 was similar, but was much lower than that of Fz5 (A) as detected by an anti-Fz5 antibody. This was probably due to the presence of the DKK moiety. (C) A diagram showing the position of the amino acid substitutions or the truncation in DKK1-Fz5 mutants. (D) DKK1-Fz5, but neither of the DKK1-Fz5 mutants, enhanced LRP6 phosphorylation. The protein level of Fz5 and DKK-Fz5 fusion proteins was detected via the Myc tag. (E) DKK1-Fz5, but neither of the two DKK1-Fz5 mutants, synergized with LRP6 to stimulate the TCF/β-catenin reporter expression in HEK293T cells.
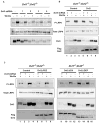
Fig. 3. Dvl is required for Wnt-induced LRP6 phosphorylation and acts via the DIX and PDZ domains
(A) Reduction of Dvl proteins diminished Wnt3a-induced Lrp6 (endogenous) phosphorylation. MEFs lacking Dvl1 and Dvl2 (Dvl1−/−;Dvl2−/−) (see Fig. S1 in the supplementary material) were infected with each of the four different lentiviral shRNAs against mouse Dvl3. After 3 days, the cells were treated with Wnt3a CM or control CM for 1 hour. Dvl3 protein level were drastically reduced by shRNA2, 3 or 4. Dvl3 protein exhibited typical Wnt-induced mobility shift (due to phosphorylation). shRNA2 and 4 were used in further experiments and yielded identical results. (B) The wild-type Dvl2 rescued LRP6 (endogenous) phosphorylation in Dvl3 knockdown Dvl1−/−;Dvl2−/− MEFs. Dvl1−/−;Dvl2−/− MEFs stably expressing Dvl2 (lanes 5 to 8) or the control vector (lanes 1 to 4) were pooled and infected with the lentiviral Dvl3 shRNA as indicated, then treated with Wnt3a or control CM for 1 hour as indicated. The exogenously expressed Dvl2 was detected by the Flag tag. (C) Dvl2ΔDEP, but neither Dvl2ΔDIX nor Dvl2ΔPDZ, rescued the Wnt3a-induced Lrp6 (endogenous) phosphorylation in Dvl3 knockdown Dvl1−/−;Dvl2−/− MEFs. Dvl1−/−;Dvl2−/− MEFs stably expressing the control vector (lanes 1–4), Dvl2ΔDIX (lanes 9–12), Dvl2ΔPDZ (lanes 5–8) or Dvl2ΔDEP (lanes 13–16) were infected with the lentiviral Dvl3 shRNA as indicated, and treated with Wnt3a CM or control CM for 1 hour as indicated. The expression of Dvl2 mutants was detected by the Flag tag.
Fig. 4. Fz recruitment of axin to the plasma membrane via Dvl (Xdsh) in Xenopus embryonic explants
Synthetic mRNAs for axin (0.5 ng), Xdsh (Dvl) or XdshΔN or Xdsh-GFP (1 ng), and human Fz5 (1 ng) were injected alone or in combinations as indicated into the Xenopus embryo. Axin and Fz proteins were detected by anti-Flag and anti-Fz5 antibodies, respectively. (A) Axin alone. (Ba–c) Xdsh-GFP plus axin. (Ca–c) Fz5 plus axin. (Da–d) Fz5 plus axin plus Xdsh-GFP. (Ea–c) Fz5 plus axin plus Xdsh. (Fa–c) Fz5 plus axin plus XdshΔN.
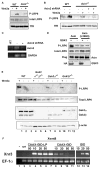
Fig. 5. Axin is required for Lrp6 phosphorylation via its ability to bind Gsk3, and inhibition of Gsk3 at the plasma membrane blocks Wnt/β-catenin signaling
(A) Axin−/− and the wild-type ES cells were treated with Wnt3a or control CM. Wnt3a-induced Lrp6 (endogenous) phosphorylation was significantly reduced in Axin−/− ES cells. (B, C) Reducing Axin2 expression in Axin−/− ES cells further inhibited Lrp6 (endogenous) phosphorylation (B). Axin−/− ES cells were infected with lentiviral shRNAs against mouse Axin2. TfR, transferin receptor, loading control. The efficiency of Axin2 mRNA knockdown was assessed by RT-PCR analysis. GAPDH, a loading control (C). (D) The wild-type axin, but not the GSK3 binding mutant axin (L396Q) promoted LRP6 phosphorylation by GSK3. Axin and axin (L396Q) were cotransfected with VSVG-tagged LRP6 in the presence or absence of GSK3 in HEK293T cells. Axin and GSK3 were detected by the Flag and HA tags, respectively. (E) Gsk3α and Gsk3β share redundant function in Wnt-induced LRP6 phosphorylation. ES cells null for both Gsk3α and Gsk3β (Gsk3α−/−;β−/−), null for either Gsk3α (Gsk3α−/−) or Gsk3β (Gsk3β−/−) and the control wild-type ES cells were treated with Wnt3a or control CM for 1 hour. The endogenous Lrp6 was examined. β-actin, loading control. (F) A plasma membrane-targeted CAAX-GID blocked wnt8 (Xwnt8) signaling in Xenopus embryo explants. GID induced nr3 (Xnr3) expression; CAAX-GID, but not CAAX-GID-LP, blocked wnt8 (Xwnt8)-induced nr3 expression (each was injected at 10–50 pg mRNA/embryo). wnt8 was injected at 10 pg mRNA/embryo. –, without reverse transcriptase; Un, uninjected control embryo; EF-1α; loading control.
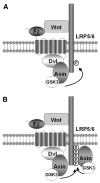
Fig. 6. A sequential recruitment and amplification model for Wnt-induced Lrp6 phosphorylation
(A) Initiation. Wnt-induced Fz-Lrp6 complex formation promotes initial Lrp6 phosphorylation via Dvl recruitment of the axin-Gsk3 complex. (B) Amplification. Initial Lrp6 phosphorylation provides docking sites and thereby recruits additional Axin-Gsk3 complex to promote further Lrp6 phosphorylation in cis and possibly in trans if/when Lrp6 multimerizes. See Discussion for details. For clarity, β-catenin, CK1 and other proteins are omitted from the axin complex, the protein composition of which may be different with and without Wnt stimulation.
Similar articles
- LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin.
Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. Cselenyi CS, et al. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8032-7. doi: 10.1073/pnas.0803025105. Epub 2008 May 28. Proc Natl Acad Sci U S A. 2008. PMID: 18509060 Free PMC article. - Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation.
Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Bilic J, et al. Science. 2007 Jun 15;316(5831):1619-22. doi: 10.1126/science.1137065. Science. 2007. PMID: 17569865 - Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled.
Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, Noutsou M, Rüdiger SG, Schwamborn K, Schambony A, Maurice MM. Tauriello DV, et al. Proc Natl Acad Sci U S A. 2012 Apr 3;109(14):E812-20. doi: 10.1073/pnas.1114802109. Epub 2012 Mar 12. Proc Natl Acad Sci U S A. 2012. PMID: 22411803 Free PMC article. - Modulation of Wnt signaling by Axin and Axil.
Kikuchi A. Kikuchi A. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review. - Dishevelled: The hub of Wnt signaling.
Gao C, Chen YG. Gao C, et al. Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review.
Cited by
- Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles.
Dobrowolski R, De Robertis EM. Dobrowolski R, et al. Nat Rev Mol Cell Biol. 2011 Nov 23;13(1):53-60. doi: 10.1038/nrm3244. Nat Rev Mol Cell Biol. 2011. PMID: 22108513 Free PMC article. Review. - Bioactivity, Molecular Mechanism, and Targeted Delivery of Flavonoids for Bone Loss.
Sharma AR, Lee YH, Bat-Ulzii A, Chatterjee S, Bhattacharya M, Chakraborty C, Lee SS. Sharma AR, et al. Nutrients. 2023 Feb 12;15(4):919. doi: 10.3390/nu15040919. Nutrients. 2023. PMID: 36839278 Free PMC article. Review. - Roles of Non-Canonical Wnt Signalling Pathways in Bone Biology.
Lojk J, Marc J. Lojk J, et al. Int J Mol Sci. 2021 Oct 7;22(19):10840. doi: 10.3390/ijms221910840. Int J Mol Sci. 2021. PMID: 34639180 Free PMC article. Review. - Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins.
Tejeda-Muñoz N, Albrecht LV, Bui MH, De Robertis EM. Tejeda-Muñoz N, et al. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10402-10411. doi: 10.1073/pnas.1903506116. Epub 2019 May 6. Proc Natl Acad Sci U S A. 2019. PMID: 31061124 Free PMC article. - Toggling a conformational switch in Wnt/β-catenin signaling: regulation of Axin phosphorylation. The phosphorylation state of Axin controls its scaffold function in two Wnt pathway protein complexes.
Tacchelly-Benites O, Wang Z, Yang E, Lee E, Ahmed Y. Tacchelly-Benites O, et al. Bioessays. 2013 Dec;35(12):1063-70. doi: 10.1002/bies.201300101. Epub 2013 Sep 19. Bioessays. 2013. PMID: 24105937 Free PMC article. Review.
References
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. - PubMed
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. - PubMed
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases