Studies on anticancer activities of antimicrobial peptides - PubMed (original) (raw)
Review
Studies on anticancer activities of antimicrobial peptides
David W Hoskin et al. Biochim Biophys Acta. 2008 Feb.
Abstract
In spite of great advances in cancer therapy, there is considerable current interest in developing anticancer agents with a new mode of action because of the development of resistance by cancer cells towards current anticancer drugs. A growing number of studies have shown that some of the cationic antimicrobial peptides (AMPs), which are toxic to bacteria but not to normal mammalian cells, exhibit a broad spectrum of cytotoxic activity against cancer cells. Such studies have considerably enhanced the significance of AMPs, both synthetic and from natural sources, which have been of importance both for an increased understanding of the immune system and for their potential as clinical antibiotics. The electrostatic attraction between the negatively charged components of bacterial and cancer cells and the positively charged AMPs is believed to play a major role in the strong binding and selective disruption of bacterial and cancer cell membranes, respectively. However, it is unclear why some host defense peptides are able to kill cancer cells when others do not. In addition, it is not clear whether the molecular mechanism(s) underlying the antibacterial and anticancer activities of AMPs are the same or different. In this article, we review various studies on different AMPs that exhibit cytotoxic activity against cancer cells. The suitability of cancer cell-targeting AMPs as cancer therapeutics is also discussed.
Figures
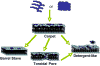
Figure 1. Models of AMP-induced membrane permeabilization
Cationic linear antimicrobial peptides are initially unstructured monomers (most AMPs) or helical and aggregated (for example, LL-37) in aqueous solution. They bind to negatively charged membrane surface by electrostatic interactions. In the carpet model, peptides bind to phospholipid head groups and align themselves parallel to the membrane surface in a carpet-like fashion until a critical threshold concentration is reached. In the barrel stave model, peptides self aggregate in the membrane once a critical threshold concentration of peptide is reached, resulting in the formation of a transmembrane pore lined by peptide oriented such that the hydrophilic face forms the inner channel while the hydrophobic face is on the outside. Since an AMP has to span the entire thickness of the lipid bilayer in this model, a minimum of ~20 residues for α-helical peptide and ~8 residues for a β-sheet peptide is required to function via the barrel stave mechanism. The toroidal pore model is an extension of the barrel stave model and postulates that at some critical concentration of peptide curvature strain induces the membranes to curve inward, resulting in the formation of a pore that is lined by both peptide and lipid headgroups. Repulsive interactions between the positively charged residues of the peptide are minimized due to the presence of the negatively charged phospholipids in the pore-lining. While the formation of these pores depend on the lipid:peptide ration and the ionic selectivity depend on the membrane composition, the lifetime of these pores seem to vary. The detergent-like model proposes that peptides intercalate in between the phospholipid head groups in a cone-like fashion causing curvature strain and micellization at local regions of high peptide density or when preformed peptide aggregates interact with lipid membranes. Recent NMR studies suggested that after sufficient time (may be a month or longer) AMPs fragment the lipid bilayers (even those containing toroidal pores) to form bicelle or micelle-like structures. Other membrane-disruptive and non-membrane disruptive mechanisms of some AMPs are discussed in the text.
Figure 2
(A) N-glycan di-N-acetyl-chitobiose trimannosyl core; (B) bisecting GlcNAc via addition of GlcNAc by GnT-III.
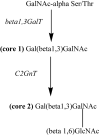
Figure 3
MUC1 O-glycan cores: non-cancerous (core 1 based) versus cancerous cells (core 2 based).
Figure 4
Reduction of tumor size by a synthetic anticancer peptide, D-K6L9: male mice without (left) and with treatment using the peptide (right) [206].
Similar articles
- Tryptophan-Rich and Proline-Rich Antimicrobial Peptides.
Mishra AK, Choi J, Moon E, Baek KH. Mishra AK, et al. Molecules. 2018 Apr 2;23(4):815. doi: 10.3390/molecules23040815. Molecules. 2018. PMID: 29614844 Free PMC article. Review. - Intracellular biomass flocculation as a key mechanism of rapid bacterial killing by cationic, amphipathic antimicrobial peptides and peptoids.
Chongsiriwatana NP, Lin JS, Kapoor R, Wetzler M, Rea JAC, Didwania MK, Contag CH, Barron AE. Chongsiriwatana NP, et al. Sci Rep. 2017 Dec 1;7(1):16718. doi: 10.1038/s41598-017-16180-0. Sci Rep. 2017. PMID: 29196622 Free PMC article. - Properties and applications of antimicrobial peptides in biodefense against biological warfare threat agents.
Dawson RM, Liu CQ. Dawson RM, et al. Crit Rev Microbiol. 2008;34(2):89-107. doi: 10.1080/10408410802143808. Crit Rev Microbiol. 2008. PMID: 18568863 Review. - Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat.
Tripathi AK, Kumari T, Tandon A, Sayeed M, Afshan T, Kathuria M, Shukla PK, Mitra K, Ghosh JK. Tripathi AK, et al. Acta Biomater. 2017 Jul 15;57:170-186. doi: 10.1016/j.actbio.2017.05.007. Epub 2017 May 5. Acta Biomater. 2017. PMID: 28483698 - Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications.
Deslouches B, Di YP. Deslouches B, et al. Oncotarget. 2017 Jul 11;8(28):46635-46651. doi: 10.18632/oncotarget.16743. Oncotarget. 2017. PMID: 28422728 Free PMC article. Review.
Cited by
- Development of a lytic peptide derived from BH3-only proteins.
Liu Q, Zhao H, Jiang Y, Wu M, Tian Y, Wang D, Lao Y, Xu N, Li Z. Liu Q, et al. Cell Death Discov. 2016 Mar 7;2:16008. doi: 10.1038/cddiscovery.2016.8. eCollection 2016. Cell Death Discov. 2016. PMID: 27551502 Free PMC article. - A Review: The Antiviral Activity of Cyclic Peptides.
Chia LY, Kumar PV, Maki MAA, Ravichandran G, Thilagar S. Chia LY, et al. Int J Pept Res Ther. 2023;29(1):7. doi: 10.1007/s10989-022-10478-y. Epub 2022 Dec 1. Int J Pept Res Ther. 2023. PMID: 36471676 Free PMC article. Review. - A coarse-grained approach to studying the interactions of the antimicrobial peptides aurein 1.2 and maculatin 1.1 with POPG/POPE lipid mixtures.
Balatti GE, Martini MF, Pickholz M. Balatti GE, et al. J Mol Model. 2018 Jul 17;24(8):208. doi: 10.1007/s00894-018-3747-z. J Mol Model. 2018. PMID: 30019106 - Bioavailability of metalloporphyrin-based SOD mimics is greatly influenced by a single charge residing on a Mn site.
Spasojevic I, Kos I, Benov LT, Rajic Z, Fels D, Dedeugd C, Ye X, Vujaskovic Z, Reboucas JS, Leong KW, Dewhirst MW, Batinic-Haberle I. Spasojevic I, et al. Free Radic Res. 2011 Feb;45(2):188-200. doi: 10.3109/10715762.2010.522575. Epub 2010 Oct 13. Free Radic Res. 2011. PMID: 20942564 Free PMC article. - Accelerating the Discovery of Anticancer Peptides through Deep Forest Architecture with Deep Graphical Representation.
Yao L, Li W, Zhang Y, Deng J, Pang Y, Huang Y, Chung CR, Yu J, Chiang YC, Lee TY. Yao L, et al. Int J Mol Sci. 2023 Feb 21;24(5):4328. doi: 10.3390/ijms24054328. Int J Mol Sci. 2023. PMID: 36901759 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LAG, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. - PubMed
- Renan MJ. How many mutations are required for tumorigenesis? Implications from human cancer cells. Mol Carcinog. 1993;7:139–146. - PubMed
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
- Espinosa E, Zamora P, Feliu J, Gonzalez Baron M. Classification of anticancer drugs-a new system based on therapeutic targets. Cancer Treat Rev. 2003;29:515–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI054515/AI/NIAID NIH HHS/United States
- R01 AI054515-03/AI/NIAID NIH HHS/United States
- S10 RR023597-01/RR/NCRR NIH HHS/United States
- AI054515/AI/NIAID NIH HHS/United States
- R01 AI054515-04/AI/NIAID NIH HHS/United States
- S10 RR023597/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources