The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response - PubMed (original) (raw)
The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response
Sohee Kwon et al. Genes Dev. 2007.
Abstract
An essential part of the cellular response to environmental stress is a reversible translational suppression, taking place in dynamic cytoplasmic structures called stress granules (SGs). We discovered that HDAC6, a cytoplasmic deacetylase that acts on tubulin and HSP90 and also binds ubiquitinated proteins with high affinity, is a novel critical SG component. We found that HDAC6 interacts with another SG protein, G3BP (Ras-GTPase-activating protein SH3 domain-binding protein 1), and localizes to SGs under all stress conditions tested. We show that pharmacological inhibition or genetic ablation of HDAC6 abolishes SG formation. Intriguingly, we found that the ubiquitin-binding domain of HDAC6 is essential and that SGs are strongly positive for ubiquitin. Moreover, disruption of microtubule arrays or impairment of motor proteins also prevents formation of SGs. These findings identify HDAC6 as a central component of the stress response, and suggest that it coordinates the formation of SGs by mediating the motor-protein-driven movement of individual SG components along microtubules.
Figures
Figure 1.
HDAC6 associates with G3BP. (A) Coimmunoprecipitation assay. 293T cells were cotransfected with an empty expression vector, Flag-tagged HDAC6, or HA-tagged G3BP. Interaction was measured by immunoprecipitation with an anti-HA or anti-Flag antibody, followed by immunoblotting with an antibody to detect G3BP or HDAC6, as indicated. Ten percent of total cell lysates used in immunoprecipitation are shown as input. (B) GST pull-down assay. Equal amounts of extracts from 293T cells transiently transfected with a vector encoding Flag-HDAC6 were incubated with beads loaded with GST alone or a GST-G3BP fusion protein. After washing, bound proteins were run on SDS-PAGE, and retained HDAC6 was detected by Western blotting using an anti-Flag antibody. (C) Coimmunoprecipitation assay with endogenous proteins. 293T cell extracts were immunoprecipitated with an anti-HDAC6 or anti-G3BP antibody, followed by immunoblotting with the indicated antibodies. (D) Extracts from 293T cells transfected with Flag-tagged HDACs and GFP-tagged G3BP were immunoprecipitated with an anti-Flag antibody and immunoblotted for G3BP.
Figure 2.
HDAC-6 interacts with G3BP via the HDAC domains, and phosphorylation of G3BP modulates its interaction with HDAC6. (A) Schematic representation of the N-terminally HA-tagged HDAC6 deletion constructs and C-terminally Myc-tagged G3BP truncated mutants used in this study. The two G3BP serine residues that can be phosphorylated are indicated. (B–G) Coimmunoprecipitation assays. One representative experiment is presented (n = 3–4). (B) HDAC6 interacts with G3BP through the HDAC domains. (Lanes 2_–_8) 293T cells were cotransfected with the indicated HDAC6 expression vectors together with a G3BP full-length expression vector, and cellular extracts were prepared. Expression of HDAC6 or G3BP was measured by Western blot with an anti-HA or anti-G3BP antibody. Association with HDAC6 was measured by performing an immunoprecipitation with an anti-HA antibody, followed by analysis of the precipitate by Western blotting with the anti-G3BP antibody. (C) G3BP interacts with HDAC6 through the acidic B domain. The domain in G3BP required to interact with HDAC6 was identified by testing extracts from cells transfected with full-length HDAC6 and deletion mutants of G3BP. Analysis was done as in B. (D) The catalytic domains of HDAC6 are not critical for interaction with G3BP. Extracts from 293T cells cotransfected with HA-tagged G3BP and the indicated Flag-tagged HDAC6 point mutants were immunoprecipitated with an anti-G3BP antibody. In the HDAC6 mutant proteins, the histidine at position 216 or 611 was mutated to alanine (H216A, H611A). (DM) Double mutant protein. (E) G3BP phosphorylation at Ser149 modulates interaction with HDAC6. GFP-fused wild-type or point mutants G3BP and Flag-tagged HDAC6 were cotransfected into 293T cells, and lysates were analyzed by immunoprecipitation and Western blotting. The lysates were immunoprecipitated with an anti-Flag antibody, followed by immunoblotting with an anti-Flag antibody, and were then reblotted for G3BP. Ten percent of total cell lysates used in immunoprecipitation are shown as input. (F) Arsenite-induced G3BP dephosphorylation promotes interaction with HDAC6. (Lanes 2,4) 293T cells were mock-transfected or transfected with HA-tagged G3BP and were treated with 1 mM arsenite for 1 h prior to lysis; extracts were then used for coimmunoprecipitation with an anti-G3BP antibody and analysis by immunoblotting, as indicated. (G) Phosphatase inhibition reduces the interaction between G3BP and HDAC6. 293T cells were cotransfected with HA-tagged G3BP and Flag-tagged HDAC6 (lanes 2–5) and treated with phosphatase inhibitors (lanes 3,4); alternatively, cell lysates were incubated in vitro with λ phosphatase (lane 5). Subsequent analysis was carried out as in E. (H) 293T cells (lanes 1_–_3,5) or extracts thereof (lane 4) were treated as indicated, and extracts were prepared and immunoprecipitated with an anti-G3BP antibody. The precipitate was then immunoblotted with anti-G3BP antibody and with an anti-phosphoserine antibody. (Ars) Arsenite; (Oka) okadaic acid; (Van) orthovanadate; (λ Ppase) λ phosphatase.
Figure 3.
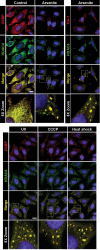
HDAC6 localizes to SGs. Exponentially growing HeLa cells were control-treated or stressed by exposure to 1 mM arsenite for 1 h, 100 mJ of UV irradiation, 1 μM CCCP for 90 min, or heat (44°C) for 1 h. Subsequently, cells were fixed and stained for G3BP, HDAC6, or TIA-1. Double-immunofluorescence experiments were performed using anti-HDAC6 and anti-G3BP or anti-TIA-1/TIAR antibody and labeled secondary antibodies (Alexa Fluor 488 [green], and Alexa Fluor 594 [red]). Nuclei were counterstained using DAPI (blue). Localization of proteins was monitored by confocal microscopy. Yellow represents colocalization. Enlargements of boxed regions are shown in the bottom row. Bar, 10 μm.
Figure 4.
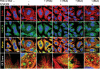
The deacetylase activity of HDAC6 is critical for assembly of SGs. HDAC inhibition impairs formation of SGs. HeLa cells were control-treated or treated with 500 nM TSA or 5 mM Butyrate for 4 h prior to treatment with arsenite for 1 h and fixation. Double-immunostaining experiments were carried out with anti-Ac-α-tubulin and anti-HDAC6 antibodies, and analysis was done by confocal microscopy. Enlargements of boxed regions are shown in the bottom row. Bar, 10 μm.
Figure 5.
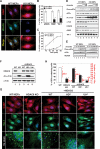
MEFs lacking HDAC6 exhibit impaired SG assembly. (A) Absence of SGs in MEFs lacking HDAC6. Wild-type or HDAC6−/y cells were exposed to 1 mM arsenite for 1 h prior to fixation and immunostaining for G3BP (red) and HDAC6 (green). The same results are obtained when staining for TIA-1 (not shown). Bar, 10 μm. (B,C) Quantification of the percentage of cells containing SGs in wild-type and KO MEFs under stress. The average percentage of SG-containing cells is indicated (histogram), as well the antibodies used for their detection (at the bottom). For the time-course experiment presented in C, MEFs were treated with arsenite for the indicated times and SGs were determined on the basis of G3BP staining. Error bars represent the standard deviation (SD) calculated from 200 cells in nine random fields. Student’s _t_-test was used for statistical analysis; (*) P < 0.01 versus wild-type control. (_D_) HDAC6 is required for SG assembly downstream from eIF2α phosphorylation. Wild-type or KO MEFs were control-treated (lanes _1_,_5_) or treated for 1 h with 0.25 mM (lanes _2_,_6_), 0.5 mM (lanes _3_,_7_), or 1 mM arsenite (lanes _4_,_8_), and extracts were prepared. Blots were probed for phospho-eIF2α, eIF2α, TIA-1/TIAR, HDAC6, G3BP, Ac-α-tubulin, and α-tubulin (as a loading control). (_E_) The kinetic of eIF2α phosphorylation is not influenced by HDAC6. Wild-type or _HDAC6−/y_ MEFs were control-treated or treated with 1 mM arsenite for different time periods, and extracts were prepared and analyzed by immunoblotting for phospho-eIF-2α, eIF-2α, and HDAC6, as indicated. (_F_) _HDAC6−/y_ MEFs (lane _2_) were used to establish rescuant cell lines expressing wild-type HDAC6 (WT, lane _3_), or a catalytically dead (HDm, lane _4_) or a non-ubiquitin-binding mutant of HDAC6 (Ubm, lane _5_). (Lane _1_) Extracts from these cells as well as from wild-type MEFs (WT) were used to monitor expression of HDAC6, Ac-α-tubulin, and tubulin. (_G_) SG formation requires intact HDAC6 function. The five cell lines described above were arsenite-treated for 1 h, and SG formation was assessed by immunostaining with an antibody against G3BP or TIA-1/TIAR, as indicated. Enlargements of boxed regions are shown at the _bottom_. Bar, 15 μm. (_H_) Quantification of the percentage of cells positive for SGs and number of large SGs per cell. (Black labeling) Percentage of cells containing SGs. Error bars represent the SD calculated from 200 cells in nine random fields. Student’s _t_-test was used for statistical analysis; (*) _P_ < 0.01 versus wild-type control. (Red labeling) Number of large (size, >1 μm) SGs per cell. Twenty cells are presented, and the median is indicated. The occurrence of SGs was determined on the basis of G3BP (or TIA-1) staining.
Figure 6.
An intact microtubule network and dynein function are required for SG assembly. (A) Hela cells were treated with 0.1% DMSO (control) for 4 h, 6.6 μM Nocodazole (Noc) for 2 h, 1 mM EHNA for 1 h, or 0.5 mM vanadate (Van) for 4 h, prior to treatment for 30 min with arsenite, as indicated. Cells were fixed and immunostained for β-tubulin and HDAC6. The insert presents a higher magnification showing SGs on the microtubule network. (B) Quantification of the percentage of HeLa cells containing SGs under conditions of microtubule disruption or inhibition of dynein ATPase activity. The occurrence of SGs was estimated based on HDAC6 immunostaining. Error bars represent the SD calculated from 200 cells in nine random fields. Student’s _t_-test was used for statistical analysis. (*) P < 0.01 versus control. (Noc) Nocodazole; (Col) Colchicine; (EHNA) erythro-9-[3-(2-Hydroxynonyl)]adenine; (Van) Orthovanadate. Bar, 10 μm. (C) SG components interact with β-tubulin. 293T cells were treated without or with 1 mM arsenite for 1 h. Cell lysates were immunoprecipitated with an anti-β-tubulin antibody, and the precipitated material was immunoblotted with the indicated antibodies. Ten percent of total cell lysates used in immunoprecipitations are shown as input. (Ars) Arsenite.
Figure 7.
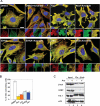
HDAC6 is not required for PB formation. HeLa cells (A) and MEFs (B) (wild-type or HDAC6−/y) were control-treated or treated with 1 mM arsenite for 1 h. Cells were then fixed, immunostained for HDAC6 or G3BP (red) and DCP1a (green), and analyzed by confocal microscopy. The squared insert presents a high magnification in the corner of the merged pictures. Bar, 5 μm. (C) Model summarizing the findings. Following cellular stress, eIF2α becomes phosphorylated and leads to stalled polysomes. HDAC6 then nucleates formation of SGs by interacting directly and indirectly with microtubules and motor proteins, G3BP and ubiquitinated proteins that are components of SGs. SGs and their precursors are depicted by red circles of various sizes, some of which are positive for ubiquitin (Ub). PBs are represented by green circles. The blue box in HDAC6 depicts the ZnF-UBP domain that binds to ubiquitin. Some of the SG components may loosely interact with microtubules; see text for details.
Similar articles
- Pseudophosphatase MK-STYX Alters Histone Deacetylase 6 Cytoplasmic Localization, Decreases Its Phosphorylation, and Increases Detyrosination of Tubulin.
Cao Y, Banks DA, Mattei AM, Riddick AT, Reed KM, Zhang AM, Pickering ES, Hinton SD. Cao Y, et al. Int J Mol Sci. 2019 Mar 22;20(6):1455. doi: 10.3390/ijms20061455. Int J Mol Sci. 2019. PMID: 30909412 Free PMC article. - HDAC6 a new cellular stress surveillance factor.
Matthias P, Yoshida M, Khochbin S. Matthias P, et al. Cell Cycle. 2008 Jan 1;7(1):7-10. doi: 10.4161/cc.7.1.5186. Epub 2007 Oct 15. Cell Cycle. 2008. PMID: 18196966 Review. - Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Bali P, et al. J Biol Chem. 2005 Jul 22;280(29):26729-34. doi: 10.1074/jbc.C500186200. Epub 2005 Jun 2. J Biol Chem. 2005. PMID: 15937340 - HDAC6 is a microtubule-associated deacetylase.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. Hubbert C, et al. Nature. 2002 May 23;417(6887):455-8. doi: 10.1038/417455a. Nature. 2002. PMID: 12024216 - The role of HDAC6 in cancer.
Aldana-Masangkay GI, Sakamoto KM. Aldana-Masangkay GI, et al. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. Epub 2010 Nov 7. J Biomed Biotechnol. 2011. PMID: 21076528 Free PMC article. Review.
Cited by
- Molecular mechanisms of stress granule assembly and disassembly.
Hofmann S, Kedersha N, Anderson P, Ivanov P. Hofmann S, et al. Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118876. doi: 10.1016/j.bbamcr.2020.118876. Epub 2020 Sep 29. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33007331 Free PMC article. Review. - Psychosocial stress and cannabinoid drugs affect acetylation of α-tubulin (K40) and gene expression in the prefrontal cortex of adult mice.
Tomas-Roig J, Ramasamy S, Zbarsky D, Havemann-Reinecke U, Hoyer-Fender S. Tomas-Roig J, et al. PLoS One. 2022 Sep 21;17(9):e0274352. doi: 10.1371/journal.pone.0274352. eCollection 2022. PLoS One. 2022. PMID: 36129937 Free PMC article. - Stress granules in cancer: Adaptive dynamics and therapeutic implications.
Jia Y, Jia R, Dai Z, Zhou J, Ruan J, Chng W, Cai Z, Zhang X. Jia Y, et al. iScience. 2024 Jun 22;27(8):110359. doi: 10.1016/j.isci.2024.110359. eCollection 2024 Aug 16. iScience. 2024. PMID: 39100690 Free PMC article. Review. - HDAC6-G3BP2 promotes lysosomal-TSC2 and suppresses mTORC1 under ETV4 targeting-induced low-lactate stress in non-small cell lung cancer.
Liu B, Zhang J, Meng X, Xie SM, Liu F, Chen H, Yao D, Li M, Guo M, Shen H, Zhang X, Xing L. Liu B, et al. Oncogene. 2023 Apr;42(15):1181-1195. doi: 10.1038/s41388-023-02641-6. Epub 2023 Feb 23. Oncogene. 2023. PMID: 36823378 - Differences between acute and chronic stress granules, and how these differences may impact function in human disease.
Reineke LC, Neilson JR. Reineke LC, et al. Biochem Pharmacol. 2019 Apr;162:123-131. doi: 10.1016/j.bcp.2018.10.009. Epub 2018 Oct 14. Biochem Pharmacol. 2019. PMID: 30326201 Free PMC article. Review.
References
- Anderson P., Kedersha N., Kedersha N. Stressful initiations. J. Cell Sci. 2002;115:3227–3234. - PubMed
- Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Kumaraswamy S., Boyapalle S., Atadja P., Boyapalle S., Atadja P., Atadja P., et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. - PubMed
- Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D., Zhang Y., Hendershot L.M., Harding H.P., Ron D., Hendershot L.M., Harding H.P., Ron D., Harding H.P., Ron D., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. - PubMed
- Bertos N.R., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Yen T.J., Khochbin S., Yang X.J., Khochbin S., Yang X.J., Yang X.J. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J. Biol. Chem. 2004;279:48246–48254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous