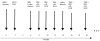Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study - PubMed (original) (raw)
Case Reports
doi: 10.1215/15228517-2007-046. Epub 2007 Dec 13.
Wei Sun, S Farzana Hussain, Mahua Dey, Lamonne Crutcher, Ken Aldape, Mark Gilbert, Samuel J Hassenbusch, Raymond Sawaya, Bob Schmittling, Gary E Archer, Duane A Mitchell, Darell D Bigner, John H Sampson
Affiliations
- PMID: 18079360
- PMCID: PMC2600844
- DOI: 10.1215/15228517-2007-046
Case Reports
Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study
Amy B Heimberger et al. Neuro Oncol. 2008 Feb.
Abstract
Cytotoxic chemotherapy that induces lymphopenia is predicted to ablate the benefits of active antitumor immunization. Temozolomide is an effective chemotherapeutic agent for patients with glioblastoma multiforme, but it induces significant lymphopenia. Although there is monthly fluctuation of the white blood cell count, specifically the CD4 and CD8 counts, there was no cumulative decline in the patient described in this case report. Depriving patients of this agent, in order to treat with immunotherapy, is controversial. Despite conventional dogma, we demonstrated that chemotherapy and immunotherapy can be delivered concurrently without negating the effects of immunotherapy. In fact, the temozolomide-induced lymphopenia may prove to be synergistic with a peptide vaccine secondary to inhibition of regulatory T cells or their delayed recovery.
Figures
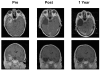
Fig. 1
Contrast-enhanced axial (top) and coronal (bottom) MR images in a patient with glioblastoma multiforme showing extent of the tumor prior to resection, after resection, and 1 year after treatment with temozolomide and immunotherapy.
Fig. 2
Photomicrograph of immunohistochemically stained operative specimen demonstrating the features indicative of glioblastoma multiforme and with positive staining for epidermal growth factor receptor variant III (original magnification, ×200).
Fig. 3
Study schema in a patient with glioblastoma multiforme (GBM) receiving temozolomide (TMZ) and the PEPvIII keyhole limpet hemocyanin (PEPvIII-KLH) vaccine. Abbreviation: XRT, radiation therapy.
Fig. 4
Flow cytometric analysis of PEPvIII-specific humoral responses induced in the peripheral blood of a patient with glioblastoma multiforme. Before the patient received any vaccine (pre), there was no detectable humoral response to PEPvIII, but after the third vaccination, there was a marked induction of PEPvIII IgG-specific responses (post). These induced humoral responses did not appear to be diminished during the sequential administration of temozolomide and the PEPvIII-keyhole limpet hemocyanin vaccine. The numbers on the x-axis denote the vaccination number; mean fluorescence intensity (MFI) is indicated on the y-axis.
Fig. 5
Flow cytometric analysis of PEPvIII-specific CD4 and CD8 T-cell responses induced in the peripheral blood of a patient with glioblastoma multiforme. To further clarify whether the temozolomide would affect the induced CD8+ cytotoxic responses to PEPvIII, the patient’s peripheral blood mononuclear cells (PBMCs) from each leukapheresis and monthly PBMCs were stimulated with the PEP-1 protein (HDTVYCVKGNKELE; 10 μg/ml) as a negative control or PEPvIII (10 μg/ml), a vaccine component. The induced immune responses were specific to the components of the vaccine and were sustained despite the sequential administration of temozolomide. As anticipated, gamma-interferon (γ-IFN) responses were initially detected, but later the patient developed PEPvIII-specific alpha-tumor necrosis factor (TNFα) responses. Abbreviations: FITC, fluorescein isothiocyanate–labeled; APC, allophycocyanin-labeled.
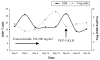
Fig. 6
Flow cytometric determination of the absolute percentage of CD8+ T cells (gated on total lymphocytes) and regulatory T cells (Treg) (CD4+CD25+Foxp3+; gated on CD4+ T cells) in the peripheral blood of a glioblastoma multiforme patient during the course of treatment with temozolomide and immunotherapy. The vaccine was administered on day 23 during this particular cycle. Abbreviation: KLH, keyhole limpet hemocyanin.
Similar articles
- Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma.
Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Sampson JH, et al. Neuro Oncol. 2011 Mar;13(3):324-33. doi: 10.1093/neuonc/noq157. Epub 2010 Dec 10. Neuro Oncol. 2011. PMID: 21149254 Free PMC article. Clinical Trial. - The evolution of the EGFRvIII (rindopepimut) immunotherapy for glioblastoma multiforme patients.
Paff M, Alexandru-Abrams D, Hsu FP, Bota DA. Paff M, et al. Hum Vaccin Immunother. 2014;10(11):3322-31. doi: 10.4161/21645515.2014.983002. Hum Vaccin Immunother. 2014. PMID: 25625931 Free PMC article. Review. - Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans.
Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Sampson JH. Mitchell DA, et al. Blood. 2011 Sep 15;118(11):3003-12. doi: 10.1182/blood-2011-02-334565. Epub 2011 Jul 18. Blood. 2011. PMID: 21768296 Free PMC article. Clinical Trial. - Myeloablative temozolomide enhances CD8⁺ T-cell responses to vaccine and is required for efficacy against brain tumors in mice.
Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, Schmittling RJ, Snyder D, Herndon JE 2nd, Bigner DD, Mitchell DA, Sampson JH. Sanchez-Perez LA, et al. PLoS One. 2013;8(3):e59082. doi: 10.1371/journal.pone.0059082. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527092 Free PMC article. - Cellular-based immunotherapies for patients with glioblastoma multiforme.
Xu X, Stockhammer F, Schmitt M. Xu X, et al. Clin Dev Immunol. 2012;2012:764213. doi: 10.1155/2012/764213. Epub 2012 Feb 28. Clin Dev Immunol. 2012. PMID: 22474481 Free PMC article. Review.
Cited by
- Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients.
Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, Li G, Liau LM, Prins RM. Fong B, et al. PLoS One. 2012;7(4):e32614. doi: 10.1371/journal.pone.0032614. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485134 Free PMC article. - Combining immunotherapy with radiation for the treatment of glioblastoma.
Chow KK, Hara W, Lim M, Li G. Chow KK, et al. J Neurooncol. 2015 Jul;123(3):459-64. doi: 10.1007/s11060-015-1762-9. Epub 2015 Apr 17. J Neurooncol. 2015. PMID: 25877468 Review. - Immunotherapy of diffuse gliomas: biological background, current status and future developments.
Grauer OM, Wesseling P, Adema GJ. Grauer OM, et al. Brain Pathol. 2009 Oct;19(4):674-93. doi: 10.1111/j.1750-3639.2009.00315.x. Brain Pathol. 2009. PMID: 19744040 Free PMC article. Review. - Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen.
King GD, Muhammad AK, Larocque D, Kelson KR, Xiong W, Liu C, Sanderson NS, Kroeger KM, Castro MG, Lowenstein PR. King GD, et al. Mol Ther. 2011 Oct;19(10):1793-801. doi: 10.1038/mt.2011.77. Epub 2011 Apr 19. Mol Ther. 2011. PMID: 21505426 Free PMC article. - Dendritic Cell Vaccination of Glioblastoma: Road to Success or Dead End.
Datsi A, Sorg RV. Datsi A, et al. Front Immunol. 2021 Nov 2;12:770390. doi: 10.3389/fimmu.2021.770390. eCollection 2021. Front Immunol. 2021. PMID: 34795675 Free PMC article. Review.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
- Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. - PubMed
- Archer G, Bigner D, Friedman A, et al. Dendritic cell vaccine for intracranial tumors I (DC Victori Trial). Paper presented at: Society for Neuro- Oncology, Education Day and Ninth Annual Scientific Meeting; 2004; Toronto, Ontario, Canada.
- Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. - PubMed
- Heimberger AB, Archer GE, Crotty LE, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50:158–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials