Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice - PubMed (original) (raw)
. 2008 Jan;118(1):183-94.
doi: 10.1172/JCI32703.
De-xiu Bu, Barry I Hudson, Jong Sun Chang, Xiaoping Shen, Kellie Hallam, Anastasia Z Kalea, Yan Lu, Rosa H Rosario, Sai Oruganti, Zana Nikolla, Dmitri Belov, Evanthia Lalla, Ravichandran Ramasamy, Shi Fang Yan, Ann Marie Schmidt
Affiliations
- PMID: 18079965
- PMCID: PMC2129235
- DOI: 10.1172/JCI32703
Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice
Evis Harja et al. J Clin Invest. 2008 Jan.
Abstract
Endothelial dysfunction is a key triggering event in atherosclerosis. Following the entry of lipoproteins into the vessel wall, their rapid modification results in the generation of advanced glycation endproduct epitopes and subsequent infiltration of inflammatory cells. These inflammatory cells release receptor for advanced glycation endproduct (RAGE) ligands, specifically S100/calgranulins and high-mobility group box 1, which sustain vascular injury. Here, we demonstrate critical roles for RAGE and its ligands in vascular inflammation, endothelial dysfunction, and atherosclerotic plaque development in a mouse model of atherosclerosis, apoE-/- mice. Experiments in primary aortic endothelial cells isolated from mice and in cultured human aortic endothelial cells revealed the central role of JNK signaling in transducing the impact of RAGE ligands on inflammation. These data highlight unifying mechanisms whereby endothelial RAGE and its ligands mediate vascular and inflammatory stresses that culminate in atherosclerosis in the vulnerable vessel wall.
Figures
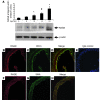
Figure 1. Expression of RAGE in the aortas of apoE–/– mice.
(A) Western blotting. At the indicated ages, aortas were retrieved from apoE–/– mice and subjected to Western blotting with anti-RAGE IgG followed by anti–β-actin IgG. RAGE/β-actin antigen relative densitometry units were calculated. *P = 0.006, #P = 0.0001, ^P < 0.0001 versus 6 weeks. (B–H) Immunohistochemistry. Aorta tissue was subjected to immunohistochemistry to detect RAGE antigen (B and F). Sites of prominent RAGE expression were confirmed to be endothelial cells, based on colocalization of RAGE expression with CD31 (C) in the merged image (D). RAGE was also expressed in smooth muscle cells, based on colocalization with smooth muscle actin (G) in the merged image (H). Staining with nonimmune IgG revealed no specific immunoreactivity (E). Original magnification, ×400.
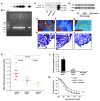
Figure 2. RAGE impacts atherosclerosis and endothelial function in apoE–/– mice.
Tg mice expressing DN-RAGE in endothelium. Founders were identified by Southern blotting (A) and by PCR (B). Lanes 1 and 6, lack of PPET DN-RAGE expression; lanes 2–5, expressing PPET DN-RAGE; C, control; M, base pair size marker. (C) Aortas were retrieved from the indicated mice and subjected to Western blotting using anti-RAGE IgG. (D) Thioglycollate-elicited macrophages were retrieved from the indicated mice and were incubated with S100b for 20 minutes. Western blotting was performed to detect phospho-JNK MAP kinase (P-JNK) followed by total JNK MAP kinase (T-JNK). Where indicated by the white line, lanes were run on the same gel but were noncontiguous. (E–J) Impact of RAGE on atherosclerosis at 14 weeks. Shown are representative images of aortic arches (E–G) and sections stained with oil red O (H–J). (K and L) Hearts were retrieved from apoE–/– (n = 12), apoE–/–RAGE–/– (n = 13), or apoE–/–Tg PPET DN-RAGE (n = 7) mice, and mean atherosclerotic lesion area (K) and lesion complexity profile (L) were determined. (M) Endothelium-dependent vasorelaxation was tested in isolated mouse aortic rings from apoE–/–, apoE–/–RAGE–/–, and apoE–/–Tg PPET DN-RAGE mice (n = 5 per group) sacrificed at 14 weeks of age. Relaxation is reported as percent of initial phenylephrine precontraction. Comparisons were conducted among groups for each agonist dose. *P < 0.05, _apoE–/–RAGE–/–_ versus _apoE–/–_ (doses >3 x 10–7 M); **P < 0.001, _apoE–/–Tg PPET DN-RAGE_ versus _apoE–/–_ (doses >10–8 M).
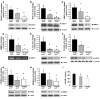
Figure 3. RAGE impacts vascular inflammation in the aortas of apoE–/– mice.
(A–H) At age 14 weeks, apoE–/–, apoE–/–RAGE–/–, and apoE–/–Tg PPET DN-RAGE mice were sacrificed and aortas retrieved. Western blotting was performed to detect VCAM-1 (A), MCP-1 (B), MMP-2 (C), IL-10 (E), CD40 (F), S100b (G), and HMGB1 (H) followed by anti–β-actin IgG. In D, zymography was performed on aorta lysates to detect activity of MMP-2. *P < 0.001 versus apoE–/–. (I) Plasma was retrieved from 14-week-old mice and subjected to ELISA for determination of soluble VCAM-1 (sVCAM-1) levels. n ≥ 4 mice per group. *P < 0.05 versus apoE–/–.
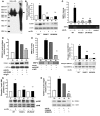
Figure 4. RAGE-mediated upregulation of inflammatory molecules in murine aortic endothelial cells: S100b.
(A) RAGE expression. Murine aortic endothelial cells from the indicated mice were subjected to Western blotting for detection of RAGE antigen. (B and C) Endothelial cells were isolated from the 3 genotypes and exposed to 10 μg/ml S100b for 4 hours. Western blotting was performed to detect VCAM-1 (B) and MMP-2 proteins (C) followed by anti–β-actin IgG. (D) Zymography for detection of MMP-2 activity was performed. (E and F) Signal transduction. Murine aortic endothelial cells from the indicated mice were incubated with 10 μg/ml S100b for 20 minutes. Western blotting for detection of phospho/total pERK and JNK MAP kinases was performed. (G and H) Endothelial cells were pretreated with the pERK MAP kinase inhibitor PD98059 (10 μM) or the JNK MAP kinase inhibitor SP600125 (10 μM) for 60 minutes prior to S100b for 20 minutes. Western blotting was performed for detection of VCAM-1 antigen. *P < 0.0001 versus unstimulated WT; **P < 0.0001 versus stimulated WT. (I) siRNA to knock down JNK MAP kinase was performed, and cells were exposed to S100b. *P < 0.0001 versus cells without S100b; **P < 0.0001 versus S100b without JNK knockdown.
Figure 5. RAGE-mediated upregulation of inflammatory molecules in murine aortic endothelial cells: oxLDL.
(A) oxLDL contains AGE epitopes. Native LDL and oxLDL (5 μg/ml) were subjected to SDS-PAGE and Western blotting using affinity-purified rabbit anti-AGE IgG. (B) Endothelial cells were incubated with 5 μg/ml oxLDL for 4 hours. Western blotting was performed for detection of VCAM-1 antigen followed by anti–β-actin IgG. (C) Zymography for detection of MMP-2 activity was performed. *P < 0.0001 versus unstimulated WT; **P < 0.0001 versus stimulated WT. (D and E) Effect of anti-AGE IgG. Murine aortic endothelial cells were pretreated with rabbit anti-AGE IgG or nonimmune rabbit IgG (nI-IgG; 50 μg/ml) for 1 hour. OxLDL (5 μg/ml) was added for 4 hours; cells were harvested and Western blotting was performed to detect VCAM-1 (D) followed by anti–β-actin IgG, or MMP-2 activity by zymography (E). (F and G) Signal transduction. Endothelial cells were incubated with 5 μg/ml oxLDL for 20 minutes. Western blotting for detection of phospho/total pERK and JNK MAP kinases was performed. (H) Endothelial cells were pretreated with the pERK MAP kinase inhibitor PD98059 (10 μM) or the JNK MAP kinase inhibitor SP600125 (10 μM) for 1 hour prior to exposure to oxLDL for 20 minutes. Western blotting was performed for detection of VCAM-1 antigen. *P < 0.0001 versus unstimulated WT; **P < 0.0001 versus oxLDL-stimulated WT.
Figure 6. RAGE-mediated upregulation of inflammatory molecules in human aortic endothelial cells.
(A) Human aortic endothelial cells were subjected to lentiviral infection introducing full-length or DN-RAGE. Cells were exposed to S100b and permeability assay performed. *P < 0.0001 versus unstimulated full-length RAGE; **P < 0.0001 versus S100b-stimulated full-length RAGE. (B and C) Human endothelial cells were subjected to introduction of siRNA to reduce RAGE transcripts (B) and RAGE protein (C) without effect on GAPDH transcripts or protein. (D) Endothelial cells were subjected to RAGE or scramble siRNA and incubated with 10 μg/ml S100b for 4 hours. Western blotting was performed to probe VCAM-1 antigen and β-actin. *P < 0.0001. (E) Human aortic endothelial cells were stimulated with 10 μg/ml S100b for 20 minutes. Western blotting was performed to detect phospho/total-JNK MAP kinases. *P < 0.0001. (F) Endothelial cells were treated with JNK MAP kinase inhibitor SP60025 (10 μM) for 60 minutes followed by S100b (10 μg/ml) for 4 hours and Western blotting performed with anti–VCAM-1 IgG and anti–β-actin IgG. **P < 0.0001 versus S100b-stimulated, non–SP600125-treated. (G) oxLDL (5 μg/ml) was incubated with human aortic endothelial cells and Western blotting performed for detection of phospho/total-JNK MAP kinase. (H) Cells were treated with 10 μM SP600125 for 1 hour followed by incubation with oxLDL for 4 hours. Western blotting was performed for detection of VCAM-1 antigen. *P < 0.0001. (I) Cells were treated with siRNA to knock down JNK MAP kinase followed by incubation with oxLDL for 4 hours. Western blotting was performed for detection of VCAM-1 antigen. Where indicated by the white line, lanes were run on the same gel but were noncontiguous. *P < 0.05; **P < 0.01.
Similar articles
- Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis.
Liu M, Yu Y, Jiang H, Zhang L, Zhang PP, Yu P, Jia JG, Chen RZ, Zou YZ, Ge JB. Liu M, et al. Acta Pharmacol Sin. 2013 Jun;34(6):830-6. doi: 10.1038/aps.2013.8. Epub 2013 Apr 8. Acta Pharmacol Sin. 2013. PMID: 23564080 Free PMC article. - RAGE and cardiovascular disease.
Park S, Yoon SJ, Tae HJ, Shim CY. Park S, et al. Front Biosci (Landmark Ed). 2011 Jan 1;16(2):486-97. doi: 10.2741/3700. Front Biosci (Landmark Ed). 2011. PMID: 21196183 Review. - Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis.
Belmokhtar K, Robert T, Ortillon J, Braconnier A, Vuiblet V, Boulagnon-Rombi C, Diebold MD, Pietrement C, Schmidt AM, Rieu P, Touré F. Belmokhtar K, et al. Arterioscler Thromb Vasc Biol. 2016 May;36(5):800-9. doi: 10.1161/ATVBAHA.115.306349. Epub 2016 Mar 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26988587 - Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response.
Yan SF, Ramasamy R, Schmidt AM. Yan SF, et al. J Mol Med (Berl). 2009 Mar;87(3):235-47. doi: 10.1007/s00109-009-0439-2. Epub 2009 Feb 3. J Mol Med (Berl). 2009. PMID: 19189073 Free PMC article. Review. - Unlocking the biology of RAGE in diabetic microvascular complications.
Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Manigrasso MB, et al. Trends Endocrinol Metab. 2014 Jan;25(1):15-22. doi: 10.1016/j.tem.2013.08.002. Epub 2013 Sep 3. Trends Endocrinol Metab. 2014. PMID: 24011512 Free PMC article. Review.
Cited by
- Targeting neutrophil: new approach against hypertensive cardiac remodeling?
Obama T, Scalia R, Eguchi S. Obama T, et al. Hypertension. 2014 Jun;63(6):1171-2. doi: 10.1161/HYPERTENSIONAHA.114.03288. Epub 2014 Apr 7. Hypertension. 2014. PMID: 24711521 Free PMC article. No abstract available. - HMGB1 in health and disease.
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. Kang R, et al. Mol Aspects Med. 2014 Dec;40:1-116. doi: 10.1016/j.mam.2014.05.001. Epub 2014 Jul 8. Mol Aspects Med. 2014. PMID: 25010388 Free PMC article. Review. - Malondialdehyde epitopes are sterile mediators of hepatic inflammation in hypercholesterolemic mice.
Busch CJ, Hendrikx T, Weismann D, Jäckel S, Walenbergh SM, Rendeiro AF, Weißer J, Puhm F, Hladik A, Göderle L, Papac-Milicevic N, Haas G, Millischer V, Subramaniam S, Knapp S, Bennett KL, Bock C, Reinhardt C, Shiri-Sverdlov R, Binder CJ. Busch CJ, et al. Hepatology. 2017 Apr;65(4):1181-1195. doi: 10.1002/hep.28970. Epub 2017 Feb 3. Hepatology. 2017. PMID: 27981604 Free PMC article. - Vascular Calcification: Current Genetics Underlying This Complex Phenomenon.
Pérez-Hernández N, Aptilon-Duque G, Blachman-Braun R, Vargas-Alarcón G, Rodríguez-Cortés AA, Azrad-Daniel S, Posadas-Sánchez R, Rodríguez-Pérez JM. Pérez-Hernández N, et al. Chin Med J (Engl). 2017 May 5;130(9):1113-1121. doi: 10.4103/0366-6999.204931. Chin Med J (Engl). 2017. PMID: 28469108 Free PMC article. Review. - Targeting the Receptor for Advanced Glycation Endproducts (RAGE): A Medicinal Chemistry Perspective.
Bongarzone S, Savickas V, Luzi F, Gee AD. Bongarzone S, et al. J Med Chem. 2017 Sep 14;60(17):7213-7232. doi: 10.1021/acs.jmedchem.7b00058. Epub 2017 May 19. J Med Chem. 2017. PMID: 28482155 Free PMC article. Review.
References
- Kislinger T.K., et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. . 1999;274:31740–31749. - PubMed
- Hofmann M.A., et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. - PubMed
- Taguchi A., et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. - PubMed
- Imanaga Y., et al. In vivo and in vitro evidence for the glycoxidation of low density lipoprotein in human atherosclerotic plaques. Atherosclerosis. . 2000;150:343–355. - PubMed
- Cybulsky M.I., Gimbrone M.A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherosclerosis. Science. . 1991;251:788–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous