Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib - PubMed (original) (raw)
. 2007 Dec 15;370(9604):2011-9.
doi: 10.1016/S0140-6736(07)61865-0.
Maria A Rupnick, Risto Kerkela, Susan M Dallabrida, David Zurakowski, Lisa Nguyen, Kathleen Woulfe, Elke Pravda, Flavia Cassiola, Jayesh Desai, Suzanne George, Jeffrey A Morgan, David M Harris, Nesreen S Ismail, Jey-Hsin Chen, Frederick J Schoen, Annick D Van den Abbeele, George D Demetri, Thomas Force, Ming Hui Chen
Affiliations
- PMID: 18083403
- PMCID: PMC2643085
- DOI: 10.1016/S0140-6736(07)61865-0
Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib
Tammy F Chu et al. Lancet. 2007.
Abstract
Background: Sunitinib, a multitargeted tyrosine-kinase inhibitor, which is approved by both US and European Commission regulatory agencies for clinical use, extends survival of patients with metastatic renal-cell carcinoma and gastrointestinal stromal tumours, but concerns have arisen about its cardiac safety. We therefore assessed the cardiovascular risk associated with sunitinib in patients with metastatic gastrointestinal stromal tumours.
Methods: We retrospectively reviewed all cardiovascular events in 75 patients with imatinib-resistant, metastatic, gastrointestinal stromal tumours who had been enrolled in a phase I/II trial investigating the efficacy of sunitinib. The composite cardiovascular endpoint was cardiac death, myocardial infarction, and congestive heart failure. We also examined sunitinib's effects on left ventricular ejection fraction (LVEF) and blood pressure. We investigated potential mechanisms of sunitinib-associated cardiac effects by studies in isolated rat cardiomyocytes and in mice.
Findings: Eight of 75 (11%) patients given repeating cycles of sunitinib in the phase I/II trial had a cardiovascular event, with congestive heart failure recorded in six of 75 (8%). Ten of 36 (28%) patients treated at the approved sunitinib dose had absolute LVEF reductions in ejection fraction (EF) of at least 10%, and seven of 36 (19%) had LVEF reductions of 15 EF% or more. Sunitinib induced increases in mean systolic and diastolic blood pressure, and 35 of 75 (47%) individuals developed hypertension (>150/100 mm Hg). Congestive heart failure and left ventricular dysfunction generally responded to sunitinib being withheld and institution of medical management. Sunitinib caused mitochondrial injury and cardiomyocyte apoptosis in mice and in cultured rat cardiomyocytes.
Interpretation: Left ventricular dysfunction might be due, in part, to direct cardiomyocyte toxicity, exacerbated by hypertension. Patients treated with sunitinib should be closely monitored for hypertension and LVEF reduction, especially those with a history of coronary artery disease or cardiac risk factors.
Conflict of interest statement
Conflicts of Interest Statement J. Desai has received speaker’s honoraria from and has served as a consultant for Pfizer. S. George has served as a consultant to Pfizer. G. Demetri has served as a consultant for Pfizer, Novartis, Bristol-Myers Squibb, Ariad, Johnson & Johnson, Genentech, Infinity Pharmaceuticals, ZymoGenetics, Alnylam, Idera, Bayer, and Serono, is a member of the Scientific Advisory Board for Plexxikon and ZioPharm, and has received research support from Daiichi-Sankyo. T. Force has served on the Speakers Bureau for Merck and Co., Inc. None of the other authors have potential conflicts of interest to disclose.
Figures
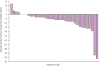
Figure 1
Maximal change in LVEF from baseline in patients treated at the FDA-approved dose. Individual data regarding absolute maximal change in LVEF from baseline to on-treatment with sunitinib 50 mg; 4 wks on/2 wks off is shown for all 36 patients. Three patients had no change in LVEF from baseline and are represented without bars.
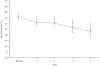
Figure 2
Model of predicted change in mean LVEF by treatment cycle. Modeling was performed using repeated-measures mixed-model regression analysis of LVEF data derived from the 36 patients treated over the first four cycles of treatment. There was a progressive decrease in mean LVEF with increasing cycles on sunitinib. The model predicts an initial 2% decline from baseline, followed by 1·5% decline per cycle for each subsequent cycle. Predicted LVEF % at baseline = 64·5; after cycle 1 = 62·4; after cycle 2 = 62·3; after cycle 3 = 60·6; and after cycle 4 = 59·4. * P=0·048 (cycle 1); * P=0·044 (cycle 2); * P=0·013 (cycle 3); ** P=0·007 (cycle 4).
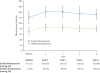
Figure 3
Effect of sunitinib 50 mg; 4 wks on/2 wks off on blood pressure. A. Changes in mean systolic (SBP) and diastolic blood pressure (DBP) during the first 4 cycles of therapy. By the first cycle, SBP and DBP had significantly increased from baseline, and remained elevated through cycle 4. B. Cumulative percentage of patients diagnosed with hypertension and on anti-hypertensive medication during the first four cycles (24 weeks) of sunitinib treatment (n=36). Hypertension was defined as > 150 mmHg systolic and/or > 100 mmHg diastolic. Grade III hypertension denotes patients who required more than one anti-hypertensive medication and/or required an increase in anti-hypertensive medication (National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0), prescribed at the discretion of the caring physician. By Cycle 4, almost half the cohort developed HTN, and 50% of patients were on anti-hypertensive medication.
Figure 3
Effect of sunitinib 50 mg; 4 wks on/2 wks off on blood pressure. A. Changes in mean systolic (SBP) and diastolic blood pressure (DBP) during the first 4 cycles of therapy. By the first cycle, SBP and DBP had significantly increased from baseline, and remained elevated through cycle 4. B. Cumulative percentage of patients diagnosed with hypertension and on anti-hypertensive medication during the first four cycles (24 weeks) of sunitinib treatment (n=36). Hypertension was defined as > 150 mmHg systolic and/or > 100 mmHg diastolic. Grade III hypertension denotes patients who required more than one anti-hypertensive medication and/or required an increase in anti-hypertensive medication (National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0), prescribed at the discretion of the caring physician. By Cycle 4, almost half the cohort developed HTN, and 50% of patients were on anti-hypertensive medication.
Figure 4
Endomyocardial biopsies obtained from two patients who developed CHF on sunitinib. Representative light photomicrographs (Patient A - top panels) show cardiomyocyte hypertrophy with mild degenerative changes and diffuse, moderate myocyte vacuolization (arrows). There was no edema, interstitial or replacement fibrosis, regional infarct or focal cell necrosis, or myocarditis or inflammation. TEM (Patient B - bottom left) demonstrates swollen, abnormal mitochondrial configurations (arrow) with effaced cristae. TEM (Patient B - bottom right) shows abundant cytoplasmic granular densities consistent with glycogen accumulation (G), membrane whorl (arrow), and lysosomal precipitates (arrowhead). Sarcomeres (S) appear well-organized and structurally normal in both patients. (Light microscopy, stained with hematoxylin and eosin, 400X; transmission EM, bar = 1 μm.)
Figure 5
Effect of sunitinib on cardiac structure, mitochondrial function and apoptosis. A. TEM of cardiac tissue from mice treated with control (left panel) or sunitinib (center and right panels) at 40 mg/kg/d for 10 days. Images from treated animals are notable for swollen mitochondria with disrupted cristae (M). Higher magnification image (right panel) illustrates membrane whorls (arrow) within the mitochondria of myocytes from sunitinib-treated mice. B. Sunitinib induces cytochrome c release from mitochondria. Neonatal rat ventricular myocytes (NRVMs) in culture were incubated in media (control) or media containing sunitinib (1 μM) for the times shown. Cells were then stained for cytochrome c. The punctate staining in the control is consistent with mitochondrial-localized cytochrome c, while the diffuse staining in the sunitinib-treated cells reflects release of cytochrome c from the mitochondria into the cytosol. C. Sunitinib activates Capase-9 and induces apoptosis. Top: NRVMs were treated with vehicle (DMSO) or sunitinib at the concentrations noted for 40 hours. Caspase-9 activity was examined. Sunitinib resulted in significant increases in caspase-9 activity at 1 μM (** P=,0·02) and 10 μM (* P=0·006). Data are mean ± SD. Bottom: Cardiomyocytes were treated with vehicle (control) or sunitinib (1 μM) for 44 hours, followed by TUNEL staining to detect apoptotic cells. * P=0·01 vs. control. D. Sunitinib induces apoptosis in vivo in the setting of increased blood pressure. Mice were fed normal chow (control) or chow containing sunitinib (10mg/kg/d) for 2 weeks. During the second week, phenylephrine (PE; 30 mg/kg/d) vs. vehicle was administered via subcutaneous mini-pump. Heart sections were then stained for TUNEL. Graph depicts percent TUNEL positive cells for the various treatments. * P=0·02 vs. control + vehicle; # P = 0·002 vs. control + PE; n = 4 for control + vehicle; n = 6 for control + PE; n = 3 for sunitinib + PE.
Figure 5
Effect of sunitinib on cardiac structure, mitochondrial function and apoptosis. A. TEM of cardiac tissue from mice treated with control (left panel) or sunitinib (center and right panels) at 40 mg/kg/d for 10 days. Images from treated animals are notable for swollen mitochondria with disrupted cristae (M). Higher magnification image (right panel) illustrates membrane whorls (arrow) within the mitochondria of myocytes from sunitinib-treated mice. B. Sunitinib induces cytochrome c release from mitochondria. Neonatal rat ventricular myocytes (NRVMs) in culture were incubated in media (control) or media containing sunitinib (1 μM) for the times shown. Cells were then stained for cytochrome c. The punctate staining in the control is consistent with mitochondrial-localized cytochrome c, while the diffuse staining in the sunitinib-treated cells reflects release of cytochrome c from the mitochondria into the cytosol. C. Sunitinib activates Capase-9 and induces apoptosis. Top: NRVMs were treated with vehicle (DMSO) or sunitinib at the concentrations noted for 40 hours. Caspase-9 activity was examined. Sunitinib resulted in significant increases in caspase-9 activity at 1 μM (** P=,0·02) and 10 μM (* P=0·006). Data are mean ± SD. Bottom: Cardiomyocytes were treated with vehicle (control) or sunitinib (1 μM) for 44 hours, followed by TUNEL staining to detect apoptotic cells. * P=0·01 vs. control. D. Sunitinib induces apoptosis in vivo in the setting of increased blood pressure. Mice were fed normal chow (control) or chow containing sunitinib (10mg/kg/d) for 2 weeks. During the second week, phenylephrine (PE; 30 mg/kg/d) vs. vehicle was administered via subcutaneous mini-pump. Heart sections were then stained for TUNEL. Graph depicts percent TUNEL positive cells for the various treatments. * P=0·02 vs. control + vehicle; # P = 0·002 vs. control + PE; n = 4 for control + vehicle; n = 6 for control + PE; n = 3 for sunitinib + PE.
Comment in
- Cardiac toxicity of sunitinib.
Joensuu H. Joensuu H. Lancet. 2007 Dec 15;370(9604):1978-80. doi: 10.1016/S0140-6736(07)61840-6. Lancet. 2007. PMID: 18083385 No abstract available. - Cardiotoxicity associated with sunitinib.
Kavsak PA, Hotte S. Kavsak PA, et al. Lancet. 2008 Apr 12;371(9620):1244; author reply 1245. doi: 10.1016/S0140-6736(08)60551-6. Lancet. 2008. PMID: 18406853 No abstract available. - Cardiotoxicity associated with sunitinib.
Hariharan S, Lowry S. Hariharan S, et al. Lancet. 2008 Apr 12;371(9620):1244-5; author reply 1245. doi: 10.1016/S0140-6736(08)60552-8. Lancet. 2008. PMID: 18406854 No abstract available. - Sunitinib-related cardiotoxicity: an interdisciplinary issue.
Ewer MS, Lenihan DJ, Khakoo AY. Ewer MS, et al. Nat Clin Pract Cardiovasc Med. 2008 Jul;5(7):364-5. doi: 10.1038/ncpcardio1222. Epub 2008 May 20. Nat Clin Pract Cardiovasc Med. 2008. PMID: 18490940 No abstract available.
Similar articles
- Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate.
Telli ML, Witteles RM, Fisher GA, Srinivas S. Telli ML, et al. Ann Oncol. 2008 Sep;19(9):1613-8. doi: 10.1093/annonc/mdn168. Epub 2008 Apr 23. Ann Oncol. 2008. PMID: 18436521 - Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis.
Di Lorenzo G, Autorino R, Bruni G, Cartenì G, Ricevuto E, Tudini M, Ficorella C, Romano C, Aieta M, Giordano A, Giuliano M, Gonnella A, De Nunzio C, Rizzo M, Montesarchio V, Ewer M, De Placido S. Di Lorenzo G, et al. Ann Oncol. 2009 Sep;20(9):1535-1542. doi: 10.1093/annonc/mdp025. Epub 2009 May 27. Ann Oncol. 2009. PMID: 19474115 - Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor.
Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, Trent J 2nd, Champion JC, Durand JB, Lenihan DJ. Khakoo AY, et al. Cancer. 2008 Jun;112(11):2500-8. doi: 10.1002/cncr.23460. Cancer. 2008. PMID: 18386829 - Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors.
Adams VR, Leggas M. Adams VR, et al. Clin Ther. 2007 Jul;29(7):1338-53. doi: 10.1016/j.clinthera.2007.07.022. Clin Ther. 2007. PMID: 17825686 Review.
Cited by
- Therapeutic potential of nitric oxide donors in the prevention and treatment of angiogenesis-inhibitor-induced hypertension.
Kruzliak P, Kovacova G, Pechanova O. Kruzliak P, et al. Angiogenesis. 2013 Apr;16(2):289-95. doi: 10.1007/s10456-012-9327-4. Epub 2012 Dec 1. Angiogenesis. 2013. PMID: 23203441 Free PMC article. Review. - Cardiotoxicity due to Chemotherapy: the Role of Biomarkers.
Stevens PL, Lenihan DJ. Stevens PL, et al. Curr Cardiol Rep. 2015 Jul;17(7):603. doi: 10.1007/s11886-015-0603-y. Curr Cardiol Rep. 2015. PMID: 26026994 Review. - Sunitinib-induced cardiac hypertrophy and the endothelin axis.
Sourdon J, Facchin C, Certain A, Viel T, Robin B, Lager F, Marchiol C, Balvay D, Yoganathan T, Favier J, Tharaux PL, Dhaun N, Renault G, Tavitian B. Sourdon J, et al. Theranostics. 2021 Feb 6;11(8):3830-3838. doi: 10.7150/thno.49837. eCollection 2021. Theranostics. 2021. PMID: 33664864 Free PMC article. - Exercise and Aerobic Fitness to Reduce Cancer-Related Cardiovascular Toxicity.
Campia U, Barac A. Campia U, et al. Curr Treat Options Cardiovasc Med. 2016 Jul;18(7):44. doi: 10.1007/s11936-016-0465-7. Curr Treat Options Cardiovasc Med. 2016. PMID: 27181399 Review. - Case report: 10-year survival of a patient with a primary hepatic gastrointestinal stromal tumor.
Lian J, Feng M, Zhang S, Lu H. Lian J, et al. Front Oncol. 2022 Dec 1;12:1035824. doi: 10.3389/fonc.2022.1035824. eCollection 2022. Front Oncol. 2022. PMID: 36530972 Free PMC article.
References
- Krause D, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. - PubMed
- Fabian MA, Biggs WH, 3rd, Treiber DK, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lélias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329–36. - PubMed
- Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–16. - PubMed
- Mann DL. Targeted cancer therapeutics: the heartbreak of success. Nat Med. 2006;12(8):881–2. - PubMed
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL61688/HL/NHLBI NIH HHS/United States
- R01 HL061688-10/HL/NHLBI NIH HHS/United States
- R01 HL067371/HL/NHLBI NIH HHS/United States
- R01 HL061688-11/HL/NHLBI NIH HHS/United States
- R01 HL061688-09/HL/NHLBI NIH HHS/United States
- R01 HL061688/HL/NHLBI NIH HHS/United States
- HL67371/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical