Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix - PubMed (original) (raw)
Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix
Pierre-Jean Wipff et al. J Cell Biol. 2007.
Abstract
The conjunctive presence of mechanical stress and active transforming growth factor beta1 (TGF-beta1) is essential to convert fibroblasts into contractile myofibroblasts, which cause tissue contractures in fibrotic diseases. Using cultured myofibroblasts and conditions that permit tension modulation on the extracellular matrix (ECM), we establish that myofibroblast contraction functions as a mechanism to directly activate TGF-beta1 from self-generated stores in the ECM. Contraction of myofibroblasts and myofibroblast cytoskeletons prepared with Triton X-100 releases active TGF-beta1 from the ECM. This process is inhibited either by antagonizing integrins or reducing ECM compliance and is independent from protease activity. Stretching myofibroblast-derived ECM in the presence of mechanically apposing stress fibers immediately activates latent TGF-beta1. In myofibroblast-populated wounds, activation of the downstream targets of TGF-beta1 signaling Smad2/3 is higher in stressed compared to relaxed tissues despite similar levels of total TGF-beta1 and its receptor. We propose activation of TGF-beta1 via integrin-mediated myofibroblast contraction as a potential checkpoint in the progression of fibrosis, restricting autocrine generation of myofibroblasts to a stiffened ECM.
Figures
Figure 1.
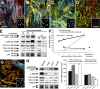
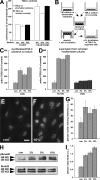
Mfs activate latent TGF-β1 from self-generated ECM depots. (A–D and G) Lung Mfs were stained for LTBP-1 (green), ED-A FN (red), α-SMA (blue), and nuclei (insets) after a 2- (A), 3- (B), or 7-d (C) culture, and after extracting all cellular components with DOC at day 7 (D). (E) Expression of LTBP-1, ED-A FN, and α-SMA was further evaluated together with TGF-β RII and LAP by Western blotting; vimentin served as loading control. Whole culture extracts were compared with samples taken after both TX-100 (TX; preserves the cytoskeleton and ECM) and DOC (preserves ECM) extraction. LTBP-1 was blotted in nonreducing conditions after digesting DOC-insoluble ECM overnight with plasmin. (F) Lung Mf (2–7 d) and subcutaneous fibroblasts (7 d) were examined for total TGF-β1. Total TGF-β1 was measured by incubating TMLC with supernatants from 80°C heat-activated trypsinized cells (cellular TGF-β1), as well as from heat-activated, DOC-insoluble ECM (ECM-associated total TGF-β1). Function-blocking TGF-β1 antibody was added to test TGF-β isoform specificity (dashed lines). TGF-β1 concentration was calculated from a standard. (G) Cultures of subcutaneous fibroblasts were stained after 7 d as in A–D. (H, left) Total culture extracts were produced from lung Mfs, lung fibroblasts (lung F), subcutaneous fibroblasts (sub F), and subcutaneous Mfs (sub Mf) and blotted as in E. Gray lines indicate that intervening lanes have been spliced out. (H, right) The DOC-insoluble fraction of 7-d Mf was used as latent TGF-β1–containing ECM for co-culture of fibroblasts or Mfs with TMLC. TGF-β1 activated by cells during 24 h was measured as luminescence compared with 24-h control cultures on plastic culture dishes. Error bars represent the SD of the mean. Bars: (A–D and G) 20 μm; (insets) 200 μm.
Figure 2.
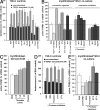
Mf contraction activates latent TGF-β1. (A) In control experiments, TMLC were incubated with various drugs for 1 h in the presence (dashed bars) or absence (solid bars) of TGF-β1. The TGF-β1 function-blocking antibody was used to assess the contribution of this isoform to the baseline luciferase level in TMLC cultures. (B) The role of Mf contraction in activating latent TGF-β1 was tested by seeding TMLC directly on 7-d-old Mf cultures 4 h before the addition of contraction-promoting drugs. An anti–TGF-β1 antibody was used to test TGF-β1 contribution in the luciferase response in Mf/TMLC co-cultures. Cell contraction inhibitors were added to contraction-stimulated and control cultures. The dotted line indicates the level of latent TGF-β1 activation by the most potent contraction agonist thrombin; the broken line shows baseline latent TGF-β activation by Mfs. Results are expressed as the percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (C) To control whether contraction agonists alone activate latent TGF-β1 from the ECM, lung Mf-derived ECM was treated for 1 h with thrombin and active TGF-β1 release into the supernatant was assessed with TMLC; ECM digested with plasmin for 1 h was used as a positive control. (D) TMLC were incubated with protease inhibitors together with contractile drugs for 1 h in the presence or absence of TGF-β1 to control interference with reporter activity. (E) Protease inhibitor cocktail was added simultaneously with contraction-promoting drugs to test whether proteases are involved in latent TGF-β1 activation by Mf contraction. Error bars represent the SD of the mean.
Figure 3.
Activation of latent TGF-β1 by Mf contraction is integrin mediated. Cultured rat lung Mfs express β3 (A), β1 (B), and αvβ5 integrin (C), as shown by confocal micrographs produced from immunostaining. (D) TMLC were seeded directly on 7-d-old Mf cultures 2 h before adding modulators of integrin binding for an additional 2 h; this was followed by another 1-h incubation in the absence or presence of 0.5 U/ml thrombin. Integrin binding was stimulated with Mn2+ and inhibited using RGD peptides, LAP–TGF-β1 (LAPβ1) decapeptide, and integrin function-blocking antibodies; scrambled DGR peptide was used as control. The broken line shows basal latent TGF-β1 activation by Mfs; the dotted line demonstrates active TGF-β1 levels after inducing contraction with thrombin only. Results are expressed as a percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (E) Controls were performed to test the effect of integrin modulators on TMLC reporter activity with and without stimulation by TGF-β1 and 0.5 U/ml thrombin. Error bars represent the SD of the mean. Bar, 10 μm.
Figure 4.
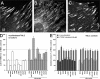
Integrin-transmitted contraction of TX-100 cytoskeletons activates latent TGF-β1. Cytoskeletons from 7-d-old Mf cultures were produced by cell extraction with TX-100. (A) Extraction efficiency was monitored by Western blotting for the plasma membrane–associated Na+/K+-ATPase. Stress fibers remaining after TX-100 extraction (B) were contracted by adding ATP for 30 min (C). Kymograph lines, 150 μm. Bar, 25 μm. (D) Isolated stress fiber contraction was visualized by a kymograph produced along the overlaid white lines in A and B. (E) TX-100 cytoskeletons were incubated for 2 h with protease inhibitors and integrin antagonists and subsequently contracted with ATP for 1 h; supernatants were analyzed for their content in active TGF-β1. Results are expressed as the percentage of nonstimulated control (BSA) corrected for TMLC baseline reporter activity. (F) To control for the influence of ATP on TMLC reporter activity, TMLC were incubated with ATP for 1 h in the presence (dashed bars) or absence (solid bars) of TGF-β1. Error bars represent the SD of the mean.
Figure 5.
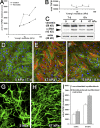
Latent TGF-β1 activation by mechanical stress requires a functional cytoskeleton. (A) To control whether stretching interferes with TGF-β1 reporting activity, TMLC were directly grown on a silicon membrane and stretched by 10% in the presence and absence of 10 pM TGF-β1. Reporter activity was compared with TMLC grown on coverslips to protect from stretching and placed upside-down on the membrane during 10% stretching. (B) This configuration was repeated in the sandwich assay, in which lung Mfs were cultured for 7 d on silicone membranes that were stretched 3–10%. After stretching, TMLC were cultured separately for further analysis. Active TGF-β1 was measured as a function of stretch applied to TMLC/Mf sandwich cultures (C), TX-100 cytoskeletons, and Mf-derived ECM (DOC; D). Active TGF-β1 is expressed as the percentage of nonstretched control (con) corrected for TMLC baseline reporter activity. Phosphorylation of Smad2 (pSmad2) was assessed by immunofluorescence detecting pSmad2 in nonstretched (E) and 10% stretched (F) conditions and by Western blotting of Mf culture extracts equilibrated for total Smad2 (H). Intensity of pSmad2 labeling in the nuclei (G) and of pSmad2 bands on the Western blot (I) was quantified by densitometric analysis. Error bars represent SD of the mean. Bar, 10 μm.
Figure 6.
Activation of latent TGF-β1 by Mf contraction increases with increasing ECM stiffness. (A) Lung Mfs were cultured for 7 d on silicone substrates with increasing stiffness (Young's modulus). TMLC reporter cells were added for a 4-h direct co-culture to assess active TGF-β1 with and without promoting Mf contraction using 0.5 U/ml thrombin. (B) Total TGF-β1 content was measured in the supernatant of heat-activated DOC-insoluble ECM produced from each substrate condition; values are expressed as luminescence in the TMLC reporter system. (C) Protein expression was evaluated by Western blotting normalized for vimentin expression and by immunostaining (D–F) for α-SMA (red), F-actin (green, phalloidin), and nuclei (blue) after 7 (D and E) and 8 h (F) of culture on 5-kPa compliant (D and F) and 47-kPa stiff (E) substrates. ECM was extracted with DOC from 7-d Mf cultures on compliant (G) and stiff (H) substrates and immunostained for LTBP-1. (I) Mfs were freshly seeded onto Mf-derived, DOC-insoluble ECM and cell-activated TGF-β1 was evaluated after 8 h of direct co-culture with TMLC as luminescence. Error bars represent the SD of the mean. Bar, 100 μm.
Figure 7.
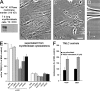
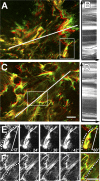
Stretching of LTBP-1 fibers by Mf contraction is reduced on compliant ECM. Mfs were grown for 8 h on 5-kPa soft (A, B, and E) and 47-kPa stiff (C, D, and F) silicone substrates provided with 7-d Mf-derived ECM. Living Mfs were incubated for 2 h with fluorescently labeled LTBP-1 antibody to follow LTBP-1 displacement by videomicroscopy. The image field includes the ECM associated with one cell. Displacement is visualized by overlaying initial LTBP-1 position (green) with LTBP-1 images taken 1 h after stimulating Mf contraction (red) with 0.5 U/ml thrombin. Dynamic deformation over 1 h was analyzed from Videos 3 and 5 (available at
http://www.jcb.org/cgi/content/full/jcb.200704042/DC1
) using kymographs (B and D) extracted along the white lines that span the long axis of one cell. Kymograph lines, 200 μm. Dotted lines on kymographs indicate fiber displacement toward the cell center on soft and rather static overall behavior on stiff substrates. Close-up frames taken every 12 min from selected fibers demonstrate fiber compression on soft (E, diagonal scale bars indicate the shortening of the fiber; Video 4) and stretching on stiff substrates (F, dotted line; Video 6). Bars, 25 μm.
Figure 8.
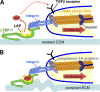
Model of Mf contraction-mediated TGF-β1 activation. The high contractile activity generated by α-SMA in stress fibers is transmitted at sites of integrins binding to RGD sites in the LAP protein as part of the LLC, which also includes TGF-β1 and LTBP-1. (A) When the LLC is anchored in a comparably stiff ECM, cell-mediated stress can induce allosteric changes in LTBP-1 and/or LAP conformation, leading to liberation of TGF-β1; such activated TGF-β1 possibly feeds back by binding to its receptor, which is located close by in the activating cell. (B) In the context of compliant ECM, the LLC is dragged toward the pulling cell but because of the lack of mechanical resistance, no conformation change occurs and TGF-β1 remains latent. Likewise, inhibition of high cell contraction and interaction of integrins with the LLC block mechanical activation of latent TGF-β1.
Similar articles
- Epigenetic regulation of myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts.
Cho JS, Moon YM, Park IH, Um JY, Moon JH, Park SJ, Lee SH, Kang HJ, Lee HM. Cho JS, et al. Clin Exp Allergy. 2012 Jun;42(6):872-82. doi: 10.1111/j.1365-2222.2011.03931.x. Clin Exp Allergy. 2012. PMID: 22239687 - Vitamin D attenuates myofibroblast differentiation and extracellular matrix accumulation in nasal polyp-derived fibroblasts through smad2/3 signaling pathway.
Lee SA, Yang HW, Um JY, Shin JM, Park IH, Lee HM. Lee SA, et al. Sci Rep. 2017 Aug 4;7(1):7299. doi: 10.1038/s41598-017-07561-6. Sci Rep. 2017. PMID: 28779150 Free PMC article. - Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction.
Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B. Sarrazy V, et al. Cardiovasc Res. 2014 Jun 1;102(3):407-17. doi: 10.1093/cvr/cvu053. Epub 2014 Mar 17. Cardiovasc Res. 2014. PMID: 24639195 Free PMC article. - Matrix Stiffness: the Conductor of Organ Fibrosis.
Santos A, Lagares D. Santos A, et al. Curr Rheumatol Rep. 2018 Jan 19;20(1):2. doi: 10.1007/s11926-018-0710-z. Curr Rheumatol Rep. 2018. PMID: 29349703 Review. - The extracellular matrix: an active or passive player in fibrosis?
Wight TN, Potter-Perigo S. Wight TN, et al. Am J Physiol Gastrointest Liver Physiol. 2011 Dec;301(6):G950-5. doi: 10.1152/ajpgi.00132.2011. Epub 2011 Apr 21. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21512158 Free PMC article. Review.
Cited by
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection.
Li C, Jiao S, Wang G, Gao Y, Liu C, He X, Zhang C, Xiao J, Li W, Zhang G, Wei B, Chen H, Wang H. Li C, et al. PLoS Pathog. 2015 Apr 24;11(4):e1004824. doi: 10.1371/journal.ppat.1004824. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25909459 Free PMC article. - Matrix, mesenchyme, and mechanotransduction.
Tschumperlin DJ. Tschumperlin DJ. Ann Am Thorac Soc. 2015 Mar;12 Suppl 1(Suppl 1):S24-9. doi: 10.1513/AnnalsATS.201407-320MG. Ann Am Thorac Soc. 2015. PMID: 25830830 Free PMC article. Review. - Alveolar epithelial disintegrity in pulmonary fibrosis.
Kulkarni T, de Andrade J, Zhou Y, Luckhardt T, Thannickal VJ. Kulkarni T, et al. Am J Physiol Lung Cell Mol Physiol. 2016 Aug 1;311(2):L185-91. doi: 10.1152/ajplung.00115.2016. Epub 2016 May 27. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27233996 Free PMC article. Review. - COVID-19 and pulmonary fibrosis: therapeutics in clinical trials, repurposing, and potential development.
Yim J, Lim HH, Kwon Y. Yim J, et al. Arch Pharm Res. 2021 May;44(5):499-513. doi: 10.1007/s12272-021-01331-9. Epub 2021 May 28. Arch Pharm Res. 2021. PMID: 34047940 Free PMC article. Review. - Adenosine/TGFβ axis in regulation of mammary fibroblast functions.
Vasiukov G, Menshikh A, Owens P, Novitskaya T, Hurley P, Blackwell T, Feoktistov I, Novitskiy SV. Vasiukov G, et al. PLoS One. 2021 Jun 8;16(6):e0252424. doi: 10.1371/journal.pone.0252424. eCollection 2021. PLoS One. 2021. PMID: 34101732 Free PMC article.
References
- Abe, M., J.G. Harpel, C.N. Metz, I. Nunes, D.J. Loskutoff, and D.B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276–284. - PubMed
- Annes, J.P., J.S. Munger, and D.B. Rifkin. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116:217–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous