Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1 - PubMed (original) (raw)
Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1
Eric J Haas-Stapleton et al. PLoS One. 2007.
Abstract
Candida albicans is an opportunistic fungal pathogen of humans that resides commensally on epithelial surfaces, but can cause inflammation when pathogenic. Resolvins are a class of anti-inflammatory lipids derived from omega-3 polyunsaturated fatty acids (PUFA) that attenuate neutrophil migration during the resolution phase of inflammation. In this report we demonstrate that C. albicans biosynthesizes resolvins that are chemically identical to those produced by human cells. In contrast to the trans-cellular biosynthesis of human Resolvin E1 (RvE1), RvE1 biosynthesis in C. albicans occurs in the absence of other cellular partners. C. albicans biosynthesis of RvE1 is sensitive to lipoxygenase and cytochrome P450 monoxygenase inhibitors. We show that 10nM RvE1 reduces neutrophil chemotaxis in response to IL-8; 1nM RvE1 enhanced phagocytosis of Candida by human neutrophils, as well as intracellular ROS generation and killing, while having no direct affect on neutrophil motility. In a mouse model of systemic candidiasis, RvE1 stimulated clearance of the fungus from circulating blood. These results reveal an inter-species chemical signaling system that modulates host immune functions and may play a role in balancing host carriage of commensal and pathogenic C. albicans.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
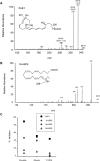
Figure 1. C. albicans produces a tri-hydroxy derivative of EPA that is structurally identical to the human anti-inflammatory lipid mediator RvE1.
The MS/MS spectra of biogenic RvE1 (1A) and 18-HEPE (1B) produced by C. albicans cultured in liquid media supplemented with EPA. (1C) C. albicans, cultured in the presence of EPA the LO inhibitors esculetin or zileuton (100 µM) as well as the CYP450 inhibitor, 17-ODA, reduced RvE1 biosynthesis (squares). LO inhibitors, but not the CYP450 inhibitor, reduced biosynthesis of 5-HEPE (circles), 12-HEPE (diamonds), and 18-HEPE (triangles).
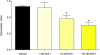
Figure 2. RvE1 blocks IL-8-stimulated neutrophil chemotaxis.
IL-8-directed chemotaxis of neutrophils was significantly inhibited by 10 and 100 nM RvE1 (Asterisks indicate significant differences from vehicle-treated controls; ANOVA: p<0.001).
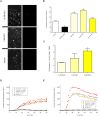
Figure 3. RvE1 enhances the effector functions of neutrophils.
(3AB) Neutrophil phagocytosis of FITC-stained heat-killed opsonized (HKO) C. albicans. (3B) Significantly more FITC-stained HKO C. albicans were phagocytosed by adherent human neutrophils treated with RvE1 (yellow bars) relative to vehicle-treated cells (open bar; asterisks indicate significant differences from vehicle-treated controls; ANOVA: p<0.001). Neutrophils treated with the non-hydrolyzable cAMP analog 8-bromo-cAMP were less likely to phagocytose the yeast (black bar). (3A) Representative images of isolated neutrophils phagocytosing FITC-stained HKO C. albicans in the presence of 10 nM RvE1, 1 nM RvE1 or vehicle and trypan blue. Green fluorescence (right panels) correspond to FITC-stained HKO C. albicans phagocytosed by neutrophils (3C) Neutrophils exposed to RvE1 exhibited a positive dose-dependent increase in their ability to kill opsonized C. albicans (yellow bars). Twice as many C. albicans were killed by neutrophils treated with 1nM RvE1 and over three times as many yeast were killed by neutrophils treated with 10 nM RvE1 relative to vehicle-treated neutrophils. Asterisks indicate significant differences from vehicle-treated controls (ANOVA: p<0.001). (3D) RvE1 had no effect on hydroxy-radical produced by neutrophils exposed to HKO C. albicans (red, orange, and yellow circles vs blue diamonds). (3E) 100 nM RvE1 increased neutrophil super-oxide production in neutrophils exposed to HKO C. albicans relative to neutrophils exposed to vehicle and HKO C. albicans (red circles vs blue diamonds) while lower concentrations of RvE1 (orange and yellow circles) did not increase neutrophil super-oxide relative to cells exposed to vehicle and HKO C. albicans (blue diamonds). 100nM RvE1 alone did not increase the amount of ROS produced by neutrophils relative to vehicle controls (3DE: black circles).
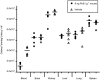
Figure 4. RvE1 reduces C. albicans concentrations in the blood.
To evaluate the actions of RvE1 on the virulence of C albicans, 8–10 week old female BALB/c mice were injected via the tail vein with RvE1 (8 ng g−1 mouse) or vehicle and C. albicans (5×104 yeast cells g−1 mouse). After 24 h, and blood and organs were collected, homogenized, serially diluted and plated. There was a 10-fold reduction in C. albicans circulating in the blood of mice injected with RvE1 compared to those injected with vehicle (circles vs triangles). In contrast, RvE1 and vehicle-treated mice showed similar levels of organ colonization.
Similar articles
- Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions.
Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Tian H, et al. Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3613-20. doi: 10.1167/iovs.08-3146. Epub 2009 May 14. Invest Ophthalmol Vis Sci. 2009. PMID: 19443724 - Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation.
Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Oh SF, et al. J Clin Invest. 2011 Feb;121(2):569-81. doi: 10.1172/JCI42545. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206090 Free PMC article. - Resolvin E1 receptor activation signals phosphorylation and phagocytosis.
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Ohira T, et al. J Biol Chem. 2010 Jan 29;285(5):3451-61. doi: 10.1074/jbc.M109.044131. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19906641 Free PMC article. - The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids.
Arita M, Clish CB, Serhan CN. Arita M, et al. Biochem Biophys Res Commun. 2005 Dec 9;338(1):149-57. doi: 10.1016/j.bbrc.2005.07.181. Epub 2005 Aug 10. Biochem Biophys Res Commun. 2005. PMID: 16112645 Review. - The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation.
Gyurko R, Van Dyke TE. Gyurko R, et al. Crit Rev Immunol. 2014;34(4):347-57. doi: 10.1615/critrevimmunol.2014009982. Crit Rev Immunol. 2014. PMID: 24941160 Free PMC article. Review.
Cited by
- Effect of inhibitors of arachidonic acid metabolism on prostaglandin E₂ production by Candida albicans and Candida dubliniensis biofilms.
Ells R, Kock JL, Albertyn J, Kemp G, Pohl CH. Ells R, et al. Med Microbiol Immunol. 2011 Feb;200(1):23-8. doi: 10.1007/s00430-010-0169-7. Epub 2010 Sep 7. Med Microbiol Immunol. 2011. PMID: 20821232 - Emerging Concepts in the Resolution of Periodontal Inflammation: A Role for Resolvin E1.
Balta MG, Loos BG, Nicu EA. Balta MG, et al. Front Immunol. 2017 Dec 14;8:1682. doi: 10.3389/fimmu.2017.01682. eCollection 2017. Front Immunol. 2017. PMID: 29312286 Free PMC article. Review. - Application of proteomics to neutrophil biology.
Luerman GC, Uriarte SM, Rane MJ, McLeish KR. Luerman GC, et al. J Proteomics. 2010 Jan 3;73(3):552-61. doi: 10.1016/j.jprot.2009.06.013. Epub 2009 Jul 4. J Proteomics. 2010. PMID: 19580889 Free PMC article. Review. - Complex and Controversial Roles of Eicosanoids in Fungal Pathogenesis.
Mendoza SR, Zamith-Miranda D, Takács T, Gacser A, Nosanchuk JD, Guimarães AJ. Mendoza SR, et al. J Fungi (Basel). 2021 Mar 28;7(4):254. doi: 10.3390/jof7040254. J Fungi (Basel). 2021. PMID: 33800694 Free PMC article. Review. - Arachidonic acid metabolites in pathogenic yeasts.
Ells R, Kock JL, Albertyn J, Pohl CH. Ells R, et al. Lipids Health Dis. 2012 Aug 8;11:100. doi: 10.1186/1476-511X-11-100. Lipids Health Dis. 2012. PMID: 22873782 Free PMC article. Review.
References
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. - PMC - PubMed
- Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clinical immunology (Orlando, Fla. 2006;119:229–240. - PubMed
- Creelman RA, Mullet JE. Biosynthesis And Action Of Jasmonates In Plants. 1997;48:355–381. - PubMed
- La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, et al. Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunological reviews. 2004;198:267–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK074448/DK/NIDDK NIH HHS/United States
- P50-DE01619/DE/NIDCR NIH HHS/United States
- DK074448/DK/NIDDK NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P01 DE07946/DE/NIDCR NIH HHS/United States
- R21 DE15290/DE/NIDCR NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
- R21 DE015290/DE/NIDCR NIH HHS/United States
- P01 DE007946/DE/NIDCR NIH HHS/United States
- GM38765/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources