Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance - PubMed (original) (raw)
Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance
Nathan J Robertson et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Although human embryonic stem (ES) cells may one day provide a renewable source of tissues for cell replacement therapy (CRT), histoincompatibility remains a significant barrier to their clinical application. Current estimates suggest that surprisingly few cell lines may be required to facilitate rudimentary tissue matching. Nevertheless, the degree of disparity between donor and recipient that may prove acceptable, and the extent of matching that is therefore required, remain unknown. To address this issue using a mouse model of CRT, we have derived a panel of ES cell lines that differ from CBA/Ca recipients at defined genetic loci. Here, we show that even expression of minor histocompatibility (mH) antigens is sufficient to provoke acute rejection of tissues differentiated from ES cells. Nevertheless, despite their immunogenicity in vivo, transplantation tolerance may be readily established by using minimal host conditioning with nondepleting monoclonal antibodies specific for the T cell coreceptors, CD4 and CD8. This propensity for tolerance could be attributed to the paucity of professional antigen-presenting cells and the expression of transforming growth factor (TGF)-beta(2). Together, these factors contribute to a state of acquired immune privilege that favors the polarization of infiltrating T cells toward a regulatory phenotype. Although the natural privileged status of ES cell-derived tissues is, therefore, insufficient to overcome even mH barriers, our findings suggest it may be harnessed effectively for the induction of dominant tolerance with minimal therapeutic intervention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
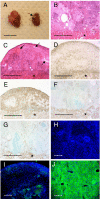
ES cell-derived tissues are rapidly rejected by fully allogeneic recipients. (A) EB differentiated from ESF75 (C57BL/6) fail to engraft under the kidney capsule of female CBA/Ca recipients (arrow), compared with syngeneic EB from ESF121. (B and C) Histological analysis of serial sections from syngeneic (B) and allogeneic (C) EB 16 days after transplantation. The asterisk identifies tissue from the recipient kidney, and arrows denote areas of tissue damage within the engrafted EB. (D–G) Serial sections from allogeneic (D and E) and syngeneic (F and G) EB stained with mAb specific for the T cell marker CD3 (D and F) or the macrophage marker, F4/80 (E and G). All micrographs are fully representative of multiple recipients in each group (n = 11 for syngeneic, and n = 12 for allogeneic donor–recipient combinations). (H–J) Expression of the MHC class I determinant H-2Kb by EB from ESF75 grafted under the kidney capsule of immunodeficient CBA.RAG1−/− mice (H) or normal CBA/Ca recipients (I), compared with sections of liver from a mouse of the H-2b haplotype, by way of a positive control (J). The distribution of H-2Kb is shown in green, and staining of nuclei by DAPI is overlaid in blue. (Scale bars: 1 cm in A, 500 μm in B–G, and 50 μm in H–J.)
Fig. 2.
Kinetics of the survival of skin grafts from each of the strains of mouse from which ES cell lines were derived, after transplantation to female CBA/Ca recipients.
Fig. 3.
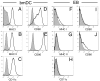
EB are devoid of professional antigen presenting cells at the time of transplantation. EB were dissociated into a single-cell suspension at day 14 of culture and were analyzed for the surface expression of DC markers by flow cytometry. Mature bmDC (A–E) and cells derived from EB (F–J) were stained for expression of MHC I (A and F), MHC II (B and G), CD11c (C and H), CD80 (D and I), and CD86 (E and J). Shaded histograms denote background staining using control, isotype-matched antibodies.
Fig. 4.
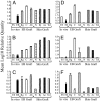
Quantitative PCR showing the relative expression of genes associated with immune privilege by ESF166 (ES; dotted bars) and by differentiated EB either in vitro (EB; black bars) or after their implantation under the kidney capsule of CBA.RAG1−/− mice (Rg; gray bars), CBA/Ca recipients (C; white bars) or CBA/Ca mice treated with mAb specific for CD4 and CD8 (Ab; hatched bars). For comparison, expression of the relevant genes is displayed for normal skin (N; diamond bars), or skin grafts that are either in the process of being rejected (Rj; checkered bars), or have been accepted spontaneously by syngeneic mice (Ac; stippled bars), or allogeneic recipients after the induction of tolerance by using mAb (Tol; striped bars). Expression of the following genes was assessed: Arginase 1 (A), Arginase 2 (B), Tgfb1 (C), Il-10 (D), Indo (E), and Tgfb2 (F). All values were normalized to expression levels of the housekeeping gene Hprt.
Fig. 5.
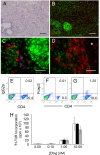
Tissues derived from ES cells provide a microenvironment conducive to the induction of CD4+Foxp3+ Treg cells. (A–D) Serial sections of teratomas derived from the male ES cell line ESF116 after implantation under the kidney capsule of female A1.RAG1−/− mice (A–C) or female A1.RAG1−/− × dnTGFβRII.RAG1−/− mice (D). Sections are stained with hematoxylin and eosin (A), CD4 (red) versus phospho-smad 2/3 (green) (B–D), and with the distribution of Foxp3 overlaid in blue (C and D), showing that expression of Foxp3 is restricted to T cells capable of responding to TGF-β2 within the local microenvironment. The asterisk identifies the recipient kidney. (Scale bars: 100 μm in A and B and 25 μm in C and D). (E–G) Flow cytometric analysis of cells purified from female (F) and male (G) EB stained for CD4 and Foxp3, compared with isotype controls (E). (H) Dose-dependent proliferative responses of splenic T cells from naïve mice (white bars) or mice receiving female (black bars) or male EB (gray bars) stimulated with Dby peptide.
Similar articles
- Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants.
Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Cobbold SP, et al. J Immunol. 2004 May 15;172(10):6003-10. doi: 10.4049/jimmunol.172.10.6003. J Immunol. 2004. PMID: 15128783 - Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells.
Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Araki R, et al. Nature. 2013 Feb 7;494(7435):100-4. doi: 10.1038/nature11807. Epub 2013 Jan 9. Nature. 2013. PMID: 23302801 - Embryonic stem cells: overcoming the immunological barriers to cell replacement therapy.
Lui KO, Waldmann H, Fairchild PJ. Lui KO, et al. Curr Stem Cell Res Ther. 2009 Jan;4(1):70-80. doi: 10.2174/157488809787169093. Curr Stem Cell Res Ther. 2009. PMID: 19149632 Review. - Immunogenicity of embryonic stem cell-derived progenitors after transplantation.
English K, Wood KJ. English K, et al. Curr Opin Organ Transplant. 2011 Feb;16(1):90-5. doi: 10.1097/MOT.0b013e3283424faa. Curr Opin Organ Transplant. 2011. PMID: 21150615 Review.
Cited by
- Dickkopf-3, a tissue-derived modulator of local T-cell responses.
Meister M, Papatriantafyllou M, Nordström V, Kumar V, Ludwig J, Lui KO, Boyd AS, Popovic ZV, Fleming TH, Moldenhauer G, Nawroth PP, Gröne HJ, Waldmann H, Oelert T, Arnold B. Meister M, et al. Front Immunol. 2015 Feb 24;6:78. doi: 10.3389/fimmu.2015.00078. eCollection 2015. Front Immunol. 2015. PMID: 25759692 Free PMC article. - Induced Pluripotent Stem Cell-Based Cancer Vaccines.
Ouyang X, Telli ML, Wu JC. Ouyang X, et al. Front Immunol. 2019 Jul 8;10:1510. doi: 10.3389/fimmu.2019.01510. eCollection 2019. Front Immunol. 2019. PMID: 31338094 Free PMC article. Review. - Multipotent adult germ-line stem cells, like other pluripotent stem cells, can be killed by cytotoxic T lymphocytes despite low expression of major histocompatibility complex class I molecules.
Dressel R, Guan K, Nolte J, Elsner L, Monecke S, Nayernia K, Hasenfuss G, Engel W. Dressel R, et al. Biol Direct. 2009 Aug 28;4:31. doi: 10.1186/1745-6150-4-31. Biol Direct. 2009. PMID: 19715575 Free PMC article. - Immunological considerations for embryonic and induced pluripotent stem cell banking.
Taylor CJ, Bolton EM, Bradley JA. Taylor CJ, et al. Philos Trans R Soc Lond B Biol Sci. 2011 Aug 12;366(1575):2312-22. doi: 10.1098/rstb.2011.0030. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21727137 Free PMC article. Review. - An emerging consensus on cardiac regeneration.
van Berlo JH, Molkentin JD. van Berlo JH, et al. Nat Med. 2014 Dec;20(12):1386-93. doi: 10.1038/nm.3764. Nat Med. 2014. PMID: 25473919 Free PMC article.
References
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
- Kehat I, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. - PubMed
- Fairchild PJ, Cartland S, Nolan KF, Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends Immunol. 2004;25:465–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials