The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma - PubMed (original) (raw)
The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma
Erin C Ward et al. Endocrinology. 2008 Apr.
Abstract
In many type I endometrial cancers, the PTEN gene is inactivated, which ultimately leads to constitutively active Akt and the inhibition of Forkhead box O1 (FOXO1), a member of the FOXO subfamily of Forkhead/winged helix family of transcription factors. The expression, regulation, and function of FOXO1 in endometrial cancer were investigated in this study. Immunohistochemical analysis of 49 endometrial tumor tissues revealed a decrease of FOXO1 expression in 95.9% of the cases compared with the expression in normal endometrium. In four different endometrial cancer cell lines (ECC1, Hec1B, Ishikawa, and RL95), FOXO1 mRNA was expressed at similar levels; however, protein levels were low or undetectable in Ecc1, Ishikawa, and RL95 cells. Using small interfering RNA technology, we demonstrated that the low levels of FOXO1 protein were due to the involvement of Skp2, an oncogenic subunit of the Skp1/Cul1/F-box protein ubiquitin complex, given that silencing Skp2 increased FOXO1 protein expression in Ishikawa cells. Inhibition of Akt in Ishikawa cells also increased nuclear FOXO1 protein levels. Additionally, progestins increased FOXO1 protein levels, specifically through progesterone receptor B (PRB) as determined by using stably transfected PRA-specific and PRB-specific Ishikawa cell lines. Finally, overexpression of triple mutant (Tm) FOXO1 in the PR-specific Ishikawa cell lines caused cell cycle arrest and significantly decreased proliferation in the presence and absence of the progestin, R5020. Furthermore, TmFOXO1 overexpression induced apoptosis in PRB-specific cells in the presence and absence of ligand. Taken together, these data provide insight into the phosphoinositide-3-kinase/Akt/FOXO pathway for the determination of progestin responsiveness and the development of alternate therapies for endometrial cancer.
Figures
Figure 1
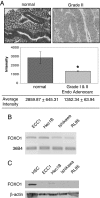
FOXO1 expression in endometrial cancer tissues and cell lines. A, Immunohistochemical staining for FOXO1 was done in normal endometrial tissue (n = 4) and grade I and II endometrial adenocarcinoma (n = 49) (magnification, ×400). Intensity of the FOXO1 stain was measured in each sample, and the unpaired t test assuming unequal variance was used to demonstrate statistical significance. *, P < 0.05. B, FOXO1 mRNA was measured in ECC1, Hec1B, Ishikawa, and RL95 cell lines using RT-PCR. The housekeeping gene, 36B4, was used as a control. The figure is representative of four experiments. C, Whole-cell lysates were subjected to Western blot analysis for FOXO1. HSCs were used as a positive control for FOXO1, and 20 μg protein were loaded per sample. The figure is representative of three experiments.
Figure 2
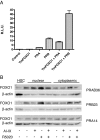
Regulation of FOXO1 by Skp2 in endometrial cancer. A, Skp2 protein levels were measured by Western blot in four endometrial cancer cell lines using HeLa cell lysates as a positive control. Twenty micrograms of protein were loaded per lane, and Skp2 antibody was used at a 1:500 dilution. The figure is representative of two experiments. B, Skp2 protein was measured after a mock transfection and transient transfection of siCONT or siSkp2 oligonucleotides in Ishikawa cells. Fifty micrograms of protein were loaded per lane, and Skp2 antibody was used at a 1:1000 dilution. The figure is representative of seven experiments. C, Immunoblotting for FOXO1 and p27 in Ishikawa whole-cell lysates mock transfected and transiently transfected with siCONT or siSkp2 was performed. Twenty micrograms of HSC cell lysate were used as a positive control for FOXO1 protein. Fifty micrograms of protein were loaded per lane. The figure is representative of seven experiments.
Figure 3
FOXO1 expression in response to an Akt inhibitor, AI-IX, in Ishikawa cells. A–C, Immunofluorescent staining for FOXO1 in Ishikawa cells was done after treatment with vehicle (A), 12 μ
m
AI-IX (B), and 24 μ
m
AI-IX (C) (magnification, ×63). Figures are representative of three experiments. D, Nuclear and cytoplasmic protein fractions were isolated from Ishikawa cells treated with 1 μ
m
AI-IX (+). Fifteen micrograms of HSC whole-cell lysate were used as a positive control for FOXO1. Forty micrograms of protein were loaded per lane for nuclear and cytoplasmic fractions. The figure is representative of five experiments.
Figure 4
Transactivation of pPRE/GRE.E1b.Luc by PR and FOXO1. A, Expression vectors for PRA or PRB were cotransfected with TmFOXO1 and the luciferase reporter pPRE/GRE.E1b.Luc in Ishikawa cells. Cells were treated with 1 μ
m
MPA, and luciferase activity was measured after 24 h. The control sample was transfected with an empty pcDNA 3.1+ vector and treated with ethanol (vehicle control) for 24 h. B, PRAB36, PRB23, and PRA14 cells were treated with 1 μ
m
AI-IX, 1 μ
m
R5020, or AI-IX and R5020 in combination. Nuclear and cytoplasmic fractions were subjected to Western blot analysis for FOXO1. Fifteen micrograms of HSC whole-cell lysate were used as a positive control for FOXO1. Forty micrograms of protein were loaded per lane for nuclear and cytoplasmic lysates. The figure is representative of two experiments.
Figure 5
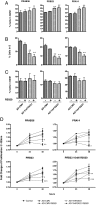
Cell cycle analysis in response to AD-TmFOXO1 overexpression and progestin treatment. PRAB36, PRB23, and PRA14 were infected with either AD-CMV (used as a control) or AD-TmFOXO1 for 24 h and then treated with 1 μ
m
R5020. After 48 h of treatment, cells were analyzed for cell cycle using PI. A–C, Graphs depict the percentage of cells in the G0/G1 phase (A), S phase (B), or G2/M phase (C) of the cell cycle. Figures represent the mean ±
sem
of three experiments. D, A cell proliferation and viability assay was conducted on PRAB36, PRB23, and PRA14 cells lines after infection with either AD-CMV or AD-TmFOXO1 for 24 h and treatment with 10 n
m
R5020 (PRB23 cells only) or 1 μ
m
R5020 for 24 and 48 h. Graphs depict the fold change of viable cells remaining after AD-TmFOXO1 infection and R5020 treatment. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Figures represent the mean ±
sem
of five experiments for the PRAB36 and PRB23 cell lines (treated at 1 μ
m
R5020), three experiments for the PRA14 cell line, and six experiments for the PRB23 cell line treated at 10 n
m
R5020.
Figure 6
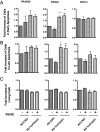
Effect of AD-TmFOXO1 and R5020 on apoptosis. PRAB36, PRB23, and PRA14 cells were infected with either AD-CMV (used as a control) or AD-TmFOXO1 for 24 h and then treated with 1 μ
m
R5020 for 48 h. Cells were analyzed by flow cytometry using annexin V/DAPI for the determination of early (A) and late (B) apoptosis and viable cells (C). Graphs depict the fold increase of cells from those infected with AD-CMV and treated with the vehicle in early and late apoptosis. *, P < 0.05; **, P < 0.01. Figures represent the mean ±
sem
of three experiments.
Similar articles
- Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1.
Hoekstra AV, Ward EC, Hardt JL, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. Hoekstra AV, et al. Gynecol Oncol. 2008 Mar;108(3):609-18. doi: 10.1016/j.ygyno.2007.11.007. Epub 2008 Jan 29. Gynecol Oncol. 2008. PMID: 18234299 - Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells.
Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EW, Bamberger AM, Ghaem-Maghami S, Brosens JJ. Goto T, et al. Oncogene. 2008 Jan 3;27(1):9-19. doi: 10.1038/sj.onc.1210626. Epub 2007 Jun 25. Oncogene. 2008. PMID: 17599040 - Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis.
Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J, Kim JJ. Neubauer NL, et al. Horm Cancer. 2011 Jun;2(3):170-81. doi: 10.1007/s12672-011-0065-7. Horm Cancer. 2011. PMID: 21760855 Free PMC article. - Regulation of FOXO protein stability via ubiquitination and proteasome degradation.
Huang H, Tindall DJ. Huang H, et al. Biochim Biophys Acta. 2011 Nov;1813(11):1961-4. doi: 10.1016/j.bbamcr.2011.01.007. Epub 2011 Jan 14. Biochim Biophys Acta. 2011. PMID: 21238503 Free PMC article. Review. - The diversity of sex steroid action: the role of micro-RNAs and FOXO transcription factors in cycling endometrium and cancer.
Lam EW, Shah K, Brosens JJ. Lam EW, et al. J Endocrinol. 2012 Jan;212(1):13-25. doi: 10.1530/JOE-10-0480. Epub 2011 Mar 7. J Endocrinol. 2012. PMID: 21382987 Review.
Cited by
- Activated mutant p110α causes endometrial carcinoma in the setting of biallelic Pten deletion.
Joshi A, Miller C Jr, Baker SJ, Ellenson LH. Joshi A, et al. Am J Pathol. 2015 Apr;185(4):1104-13. doi: 10.1016/j.ajpath.2014.12.019. Epub 2015 Feb 16. Am J Pathol. 2015. PMID: 25698082 Free PMC article. - Forkhead box J1 expression is upregulated and correlated with prognosis in patients with clear cell renal cell carcinoma.
Zhu P, Piao Y, Dong X, Jin Z. Zhu P, et al. Oncol Lett. 2015 Sep;10(3):1487-1494. doi: 10.3892/ol.2015.3376. Epub 2015 Jun 16. Oncol Lett. 2015. PMID: 26622696 Free PMC article. - Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology.
Maiese K, Hou J, Chong ZZ, Shang YC. Maiese K, et al. ScientificWorldJournal. 2009 Oct 2;9:1072-104. doi: 10.1100/tsw.2009.121. ScientificWorldJournal. 2009. PMID: 19802503 Free PMC article. Review. - Clever cancer strategies with FoxO transcription factors.
Maiese K, Chong ZZ, Shang YC, Hou J. Maiese K, et al. Cell Cycle. 2008 Dec 15;7(24):3829-39. doi: 10.4161/cc.7.24.7231. Epub 2008 Dec 21. Cell Cycle. 2008. PMID: 19066462 Free PMC article. Review. - Polymorphisms in microRNA target sites of forkhead box O genes are associated with hepatocellular carcinoma.
Tan C, Liu S, Tan S, Zeng X, Yu H, Li A, Bei C, Qiu X. Tan C, et al. PLoS One. 2015 Mar 4;10(3):e0119210. doi: 10.1371/journal.pone.0119210. eCollection 2015. PLoS One. 2015. PMID: 25739100 Free PMC article.
References
- Ryan A, Susil B, Jobling T, Oehler M 2005 Endometrial cancer. Cell Tissue Res 322:53–61 - PubMed
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ 2007 Cancer statistics, 2007. CA Cancer J Clin 57:43–66 - PubMed
- Hecht J, Mutter G 2006 Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol 24:4783–4791 - PubMed
- Kau T, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA 2003 A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4:463–476 - PubMed
- Brazil DP, Yang ZZ, Hemmings BA 2004 Advances in protein kinase B signaling: AKTion on multiple fronts. Trends Biochem Sci 29:233–242 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous