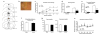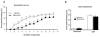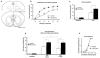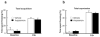The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning - PubMed (original) (raw)
The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning
Stephanie Bissière et al. Biol Psychiatry. 2008.
Abstract
Background: The rostral anterior cingulate cortex (rACC) and the amygdala consistently emerge from neuroimaging studies as brain regions crucially involved in normal and abnormal fear processing. To date, however, the role of the rACC specifically during the acquisition of auditory fear conditioning still remains unknown. The aim of this study is to investigate a possible top-down control of a specific rACC sub-region over amygdala activation during pavlovian fear acquisition.
Methods: We performed excitotoxic lesions, temporal inactivation, and activation of a specific sub-region of the rACC that we identified by tracing studies as supporting most of the connectivity with the basolateral amygdala (r(Amy)-ACC). The effects of these manipulations over amygdala function were investigated with a classical tone-shock associative fear conditioning paradigm in the rat.
Results: Excitotoxic lesions and transient inactivation of the r(Amy)-ACC pre-training selectively produced deficits in the acquisition of the tone-shock associative learning (but not context). This effect was specific for the acquisition phase. However, the deficit was found to be transient and could be overcome by overtraining. Conversely, pre-training transient activation of the r(Amy)-ACC facilitated associative learning and increased fear expression.
Conclusions: Our results suggest that a subregion of the rACC is key to gating the efficiency of amygdala-dependent auditory fear conditioning learning. Because r(Amy)-ACC inputs were confirmed to be glutamatergic, we propose that recruitment of this brain area might modulate overall basolateral amygdala excitatory tone during conditioned stimulus-unconditioned stimulus concomitant processing. In the light of clinical research, our results provide new insight on the effect of inappropriate rACC recruitment during emotional events.
Figures
Figure 1
Lesion of the rAmy-ACC produce deficit in fear acquisition. (a) Examples of the larger (light grey) and smaller (dark grey) extend of the lesions. (b) Representative phase contrast image showing a site specific lesion of the Cg1 (c) Lesion of the rAmy-ACC (black squares: n = 10) exhibit a slower acquisition time course than sham (white dots: n = 12). (d) The mean time spent freezing (± s.e.m) during the acquisition phase was significantly reduced in lesioned animals (black dots, n = 10) compared to sham (n = 12). (e) Fear expression in lesioned animals was also impaired on day 2 There was no difference during baseline habituation (no tone) between groups. (f) Lesions of the rAmy-ACC did not change locomotor activity as measured by the total immobility and total distance traveled. (g) There was no difference between the two groups in the response to the six consecutive shocks given at 0.6mA.
Figure 2
Fear learning is impaired by blocking neuronal activity in the rAmy-ACC. (a) Example of injections sites for the muscimol microinjected group. (b) Phase contrast image showing two toluedine dark blue dots indicating the location of the tip of the cannulae. (c) Inactivation of the rAmy-ACC also impaired fear acquisition. There was no effect of muscimol injection on baseline activity. (d) 24 hours after learning fear expression was also reduced without affecting habituation to a new context (no tone group). (e) Both groups showed no difference in the threshold for foot-shock induced behaviour. (f and g) Muscimol injection post-training did not affect freezing levels during fear acquisition and on day two during CS+ presentation.
Figure 3
(a) A longer training protocol could overcome the impairment in fear acquisition induced by rAmy-ACC inactivation. (b) On day two, there was no difference between the lesion and vehicle group in conditioned responses to the CS+ presentation
Figure 4
Activation of the rAmy-ACC facilitates and strengthens fear learning. (a) Example of bicuculline injection targeting the rAmy-ACC. (b) The time course of the fear learning was facilitated by neuronal activation of the rAmy-ACC. (c) There was a significant increase in the total amount of freezing across the conditioning phase in bicuculline treated group compare to vehicle. (c) On day two, freezing levels to the CS+ presentation were significantly increased in the bicuculline group compared to vehicle Bicuculline injection did not induce changes in baseline activity. Following three days of extinction protocol, the vehicle group showed significant reduction in freezing behavior to the CS+. In the contrary, the bicuculline group exhibited the same levels of freezing to the tone 24 hours and 4 days later suggesting that extinction was impaired. (d) No threshold difference in US-induced movements were observed between the vehicle and the bicuculline groups.
Figure 5
Blocking protein synthesis in the rAmy-ACC pre-training did not affect fear learning (a) or fear expression (b)
Figure 6
Connectivity between the rACC and amygdala. (a) The fluoresecent neuronal tracers FB and DiI were stereotaxically injected at different anterior-posterior positions along the ACC. Only injections at AP:+2.7, +1.7 coordinates resulted in specific labelling in the BLA. Images show injection sites in the ACC and amygdala three weeks later. Higher magnification images reveal FB-positive neuronal cell bodies and processes and punctuated DiI-positive axonal terminals. Double labelling with the nuclear markers ethidium bromide (EthBr) or Hoechst was used to indicate the position of all nuclei. (b) Coronal sections along the anterior-posterior axis of the BLA indicate the areas of FB (blue dots) and DiI (red dots) labelling. Note that the areas occupied by the two tracers and non-overlapping and that their position changes along the anterior-posterior axis of the BLA.
Figure 7
Section through the amygdala traced with either DiI or fast blue (FB) were immunolabeled with antibodies against V-GAT or V-GLUT. DiI-positive puncta do not co-localize with V-GAT (a, c), but they do with V-GLUT, co-localization is seen in yellow (b, c). a′ and b′ are higher magnification images of a and b. Most FB-positive neurons are negative for V-GAT (d, f) and express V-GLUT, co-localization in white (e, f). d′ and e′ are higher magnification images of d and e. Bar, 50 μm in a, b, d, e and 25 μm in a′, b′, d′, e′.
Similar articles
- Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning.
Chau LS, Prakapenka A, Fleming SA, Davis AS, Galvez R. Chau LS, et al. Neurobiol Learn Mem. 2013 Nov;106:127-33. doi: 10.1016/j.nlm.2013.07.010. Epub 2013 Jul 24. Neurobiol Learn Mem. 2013. PMID: 23891993 - Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response.
Jhang J, Lee H, Kang MS, Lee HS, Park H, Han JH. Jhang J, et al. Nat Commun. 2018 Jul 16;9(1):2744. doi: 10.1038/s41467-018-05090-y. Nat Commun. 2018. PMID: 30013065 Free PMC article. - Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala.
Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Malin EL, et al. Neurobiol Learn Mem. 2007 Feb;87(2):295-302. doi: 10.1016/j.nlm.2006.09.004. Epub 2006 Oct 31. Neurobiol Learn Mem. 2007. PMID: 17079169 Free PMC article. - Interplay of amygdala and cingulate plasticity in emotional fear.
Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, Koga K, Zhuo M. Toyoda H, et al. Neural Plast. 2011;2011:813749. doi: 10.1155/2011/813749. Epub 2011 Sep 7. Neural Plast. 2011. PMID: 21912749 Free PMC article. Review. - Neural circuits for a top-down control of fear and extinction.
Marek R, Sun Y, Sah P. Marek R, et al. Psychopharmacology (Berl). 2019 Jan;236(1):313-320. doi: 10.1007/s00213-018-5033-2. Epub 2018 Sep 13. Psychopharmacology (Berl). 2019. PMID: 30215217 Review.
Cited by
- Neuronal tuning to threat exposure remains stable in the mouse prefrontal cortex over multiple days.
Sylte OC, Muysers H, Chen HL, Bartos M, Sauer JF. Sylte OC, et al. PLoS Biol. 2024 Jan 11;22(1):e3002475. doi: 10.1371/journal.pbio.3002475. eCollection 2024 Jan. PLoS Biol. 2024. PMID: 38206890 Free PMC article. - Neuronal mechanism of innate rapid processing of threating animacy cue in primates: insights from the neuronal responses to snake images.
Setogawa T, Matsumoto J, Nishijo H, Nishimaru H. Setogawa T, et al. Front Psychol. 2024 Aug 29;15:1462961. doi: 10.3389/fpsyg.2024.1462961. eCollection 2024. Front Psychol. 2024. PMID: 39268378 Free PMC article. Review. - Differential reduction of gray matter volume with age in 35 cortical areas in men (more) and women (less).
Christova P, Georgopoulos AP. Christova P, et al. J Neurophysiol. 2023 Apr 1;129(4):894-899. doi: 10.1152/jn.00066.2023. Epub 2023 Mar 15. J Neurophysiol. 2023. PMID: 36922162 Free PMC article. - Conserved features of anterior cingulate networks support observational learning across species.
Burgos-Robles A, Gothard KM, Monfils MH, Morozov A, Vicentic A. Burgos-Robles A, et al. Neurosci Biobehav Rev. 2019 Dec;107:215-228. doi: 10.1016/j.neubiorev.2019.09.009. Epub 2019 Sep 8. Neurosci Biobehav Rev. 2019. PMID: 31509768 Free PMC article. Review. - Contextual Fear Memory Maintenance Changes Expression of pMAPK, BDNF and IBA-1 in the Pre-limbic Cortex in a Layer-Specific Manner.
Chaaya N, Wang J, Jacques A, Beecher K, Chaaya M, Battle AR, Johnson LR, Chehrehasa F, Belmer A, Bartlett SE. Chaaya N, et al. Front Neural Circuits. 2021 Jul 6;15:660199. doi: 10.3389/fncir.2021.660199. eCollection 2021. Front Neural Circuits. 2021. PMID: 34295224 Free PMC article.
References
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. - PubMed
- Aguado L. Neuroscience of Pavlovian conditioning: a brief review. Span J Psychol. 2003;6:155–167. - PubMed
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. - PubMed
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. - PubMed
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources