APOE genotype-specific differences in the innate immune response - PubMed (original) (raw)
APOE genotype-specific differences in the innate immune response
Michael P Vitek et al. Neurobiol Aging. 2009 Sep.
Abstract
Apolipoprotein-E protein is an endogenous immunomodulatory agent that affects both the innate and the adaptive immune responses. Since individuals with the APOE4 gene demonstrate worsened pathology and poorer outcomes in many neurological disorders, we examined isoform-specific differences in the response of microglia, the primary cellular component of the brain's innate immune response, in detail. Our data demonstrate that microglia derived from APOE4/4 targeted replacement mice demonstrate a pro-inflammatory phenotype that includes altered cell morphology, increased NO production associated with increased NOS2 mRNA levels, and higher pro-inflammatory cytokine production (TNFalpha, IL-6, IL12p40) compared to microglia derived from APOE3/3 targeted replacement mice. The effect is gene dose-dependent and increases with the number of APOE4 gene alleles. The APOE genotype-specific immune profile observed in the microglial immune response is also observed in the cortex of aged APOE3/3 and APOE4/4 mice treated with lipopolysacchride (LPS) and in peripheral (peritoneal) macrophages. To determine if APOE4's action resulted from an isoform-specific difference in effective levels of the apolipoproteins, we generated mice expressing only a single allele of APOE3. Immune-stimulated macrophages from APOE3/0 mice demonstrated an increased inflammatory response compared to APOE3/3 mice, but less than in APOE4/4 mice. These data suggest that inhibition of inflammation depends upon the dose of apoE3 protein available and that apoE4 protein may alter inflammation partly by dose effects and partly by being qualitatively different than apoE3. Overall, these data emphasize the important role of apolipoprotein E and of the APOE genotype on the immune responses that are evident in most, if not all, neurological disease.
Conflict of interest statement
Conflict of Interest Disclosure: Dr. Vitek is the founder and principal in Cognosci, Inc. Dr. Brown and Dr. Colton have no conflicts.
Figures
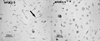
Figure 1
Differential morphology in APOE3/3 and APOE4/4 primary microglial cultures. Typical resting (non-immune stimulated) APOE3/3 (left panel) or APOE4/4 (right panel) microglial cultures were viewed under brightfield microscopy. Microglia from APOE4/4 mice were consistently observed to be less branched and demonstrated a rounded, more ameboid appearance compared to APOE3/3 microglia.
Figure 2
The presence of the APOE4 gene promotes induction of NOS2 mRNA and increased production of NO in immune stimulated microglia: Panel A- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) plus increasing doses of PIC or LPS. * P <0.04 for IFNγ–treated APOE4/4 microglia compared to IFNγ–treated APOE3/3 microglia; ** p <0.0001 for IFNγ + PIC- treated APOE4/4 microglia compared to APOE3/3 microglia; ***p <0.0001 for IFNγ + LPS - treated APOE4/4 microglia compared to APOE3/3 microglia; all using 2-way ANOVA. A significant effect of dose was observed for PIC treatment (p < 0.001; 2 way ANOVA) but was not observed for LPS treatment. Panel B- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) + LPS (100 ng/ml) in media containing physiological levels of arginine (10 µm). * p< 0.001 for APOE4/4 compared to APOE3/3 microglia. Panel C- Relative changes in mRNA for NOS2 was determined for LPS (10 ng/ml) + IFNγ (10 U/ml)-treated APOE3/3 microglia compared to APOE4/4 microglia. A significant increase (p < 0.03; unpaired student’s t test) was observed after 6 hrs of treatment in APOE4/4 compared to APOE3/3 microglia. Panel D- A significant decrease (***p < 0.001; 2-way ANOVA) in supernatant nitrite levels was found in both APOE3/3 and APOE4/4 microglia treated with LPS (100 ng/ml) + IFNγ (100 U/ml) in the presence of varying doses of 1400W, an iNOS inhibitor. No effect of 1400W alone was observed. #= p < 0.01 for LPS + IFNγ-treated APOE4/4 microglia compared to APOE3/3 microglia.
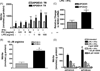
Figure 3
Cytokine production in APOE4/4 microglia compared to APOE3/3 microglia. Supernatant levels of TNFα (A); IL-6 (B); IL-12 p40 (C) and IL-4 (D) were measured after 24 hrs of treatment with LPS (100 ng/ml) + IFNγ (100 U/ml). Significantly increased levels for APOE4/4 microglia compared to APOE3/3 microglia were observed for TNFα, IL-6 and IL-12 while a significant decrease was observed for IL-4 (* = p < 0.001; unpaired student’s t test).
Figure 4
Innate immune responses are also increased in cultured peritoneal macrophages derived from adult male APOE4/4 mice. Panel A- Average supernatant levels of nitrite (± SEM) were measured for APOE3/3 or APOE4/4 peritoneal macrophage cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) plus increasing doses of PIC or LPS. Using 2-way ANOVA, a significant effect of APOE4 genotype (* p < 0.001) was observed for all treatment conditions while a significant effect of dose (p < 0.01) was observed for PIC treatment. Panel B- Average supernatant levels of TNFα (± SEM) were measured after 24 hrs in immune stimulated APOE3/3 and APOE4/4 peritoneal macrophage cultures using ELISA. LPS = 100 ng/ml; IFNγ= 100 U/ml. In each case, a significantly higher level of TNFα was found in APOE4/4 cultures compared to APOE3/3 ( * = p < 0.001; 2 way ANOVA).
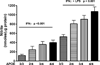
Figure 5
The effect of APOE4 is dependent on the allelic dose. Average supernatant levels of nitrite (± SEM) were measured for APOE3/3, APOE2/4, APOE3/4 or APOE4/4 microglial cultures stimulated for 24 hrs with recombinant murine IFNγ (100 U/ml) alone or plus LPS (100 ng/ml). A significant (p < 0.001, 1-way ANOVA) increase in nitrite levels was found for increasing APOE4 gene numbers under both stimulation conditions
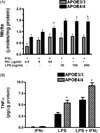
Figure 6
The gene effect of APOE4 is not solely dependent on the level of apoliporotein E protein. Panel A- Western blot depicting the expression of human apoE protein in brain (lanes 1–4; 7–12) and liver (lanes 5,6) lysates from APOE3/3 and APOE4/4 targeted replacement mice. Lower levels of protein were observed in APOE4/4 compared to APOE3/3 mice regardless of age (neonatal mice – lanes 1,2, 9,10; aged to >56 wks- all other lanes) or if immune stimulated with LPS (lanes 7,8). GAPDH was used as a protein loading control. Panel B- Western blot confirms a decreased expression of apolipoprotein E in LPS-treated APOE3/0 brain lysates compared to LPS-treated APOE3/3 brain lysates. Average relative changes in the ratio of apoE protein to GAPDH levels for each genotype are also shown (n= 4 mice/group). * = p < 0.001 for APOE3/3 brain lysates compared to either APOE4/4 or APOE3/0 lysates. Panel C/D- Average supernatant levels (± SEM) of TNFα (C) or IL-6 (D) were measured after 24hrs for immune stimulated APOE3/3 and APOE4/4 peritoneal macrophage cultures using ELISA. LPS = 50 ng/ml; IFNγ = 10 U/ml. * p < 0.001 for APOE3/3 compared to APOE3/0 cells; # p< 0.01 for APOE4/4 compared to APOE3/0 cells; ** p < 0.0001 for APOE4/4 compared to APOE3/3 cells; *** p < 0.001 for APOE4/4 compared to APOE3/3 cells and ## p < 0.001 for APOE4/4 compared to APOE3/0 cells.
Figure 7
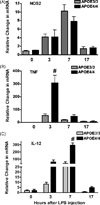
In vivo changes in the brain’s innate immune response in APOE4/4 and APOE3/3 adult mice. Adult APOE3/3 and APOE4/4 mice were injected with LPS and brains harvested at 0, 3, 7 or 17 hrs after injection. Average relative changes (± SEM) in mRNAs for NOS2 (A), for _TNF_α (B) and for IL-12 p40 (C) were determined using quantitative RT-PCR. Significantly higher levels of mRNA were observed for _TNF_α and IL-12p40 (# p < 0.001; 2 way ANOVA).
Similar articles
- Androgen-mediated immune function is altered by the apolipoprotein E gene.
Brown CM, Xu Q, Okhubo N, Vitek MP, Colton CA. Brown CM, et al. Endocrinology. 2007 Jul;148(7):3383-90. doi: 10.1210/en.2006-1200. Epub 2007 Mar 29. Endocrinology. 2007. PMID: 17395708 - APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress.
Colton CA, Brown CM, Cook D, Needham LK, Xu Q, Czapiga M, Saunders AM, Schmechel DE, Rasheed K, Vitek MP. Colton CA, et al. Neurobiol Aging. 2002 Sep-Oct;23(5):777-85. doi: 10.1016/s0197-4580(02)00016-7. Neurobiol Aging. 2002. PMID: 12392781 - Human apoE3 but not apoE4 rescues impaired astrocyte activation in apoE null mice.
Ophir G, Meilin S, Efrati M, Chapman J, Karussis D, Roses A, Michaelson DM. Ophir G, et al. Neurobiol Dis. 2003 Feb;12(1):56-64. doi: 10.1016/s0969-9961(02)00005-0. Neurobiol Dis. 2003. PMID: 12609489 - Implications of apolipoprotein E genotype on inflammation and vitamin E status.
Huebbe P, Lodge JK, Rimbach G. Huebbe P, et al. Mol Nutr Food Res. 2010 May;54(5):623-30. doi: 10.1002/mnfr.200900398. Mol Nutr Food Res. 2010. PMID: 20183830 Review. - Sex steroids, APOE genotype and the innate immune system.
Colton CA, Brown CM, Vitek MP. Colton CA, et al. Neurobiol Aging. 2005 Mar;26(3):363-72. doi: 10.1016/j.neurobiolaging.2004.08.001. Neurobiol Aging. 2005. PMID: 15639315 Review.
Cited by
- Apolipoprotein E variants correlate with the clinical presentation of paediatric inflammatory bowel disease: A cross-sectional study.
Glapa-Nowak A, Szczepanik M, Iwańczak B, Kwiecień J, Szaflarska-Popławska AB, Grzybowska-Chlebowczyk U, Osiecki M, Dziekiewicz M, Stawarski A, Kierkuś J, Banasiewicz T, Banaszkiewicz A, Walkowiak J. Glapa-Nowak A, et al. World J Gastroenterol. 2021 Apr 14;27(14):1483-1496. doi: 10.3748/wjg.v27.i14.1483. World J Gastroenterol. 2021. PMID: 33911469 Free PMC article. - 25-Hydroxycholesterol amplifies microglial IL-1β production in an apoE isoform-dependent manner.
Wong MY, Lewis M, Doherty JJ, Shi Y, Cashikar AG, Amelianchik A, Tymchuk S, Sullivan PM, Qian M, Covey DF, Petsko GA, Holtzman DM, Paul SM, Luo W. Wong MY, et al. J Neuroinflammation. 2020 Jun 17;17(1):192. doi: 10.1186/s12974-020-01869-3. J Neuroinflammation. 2020. PMID: 32552741 Free PMC article. - APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice.
Liu DS, Pan XD, Zhang J, Shen H, Collins NC, Cole AM, Koster KP, Ben Aissa M, Dai XM, Zhou M, Tai LM, Zhu YG, LaDu M, Chen XC. Liu DS, et al. Mol Neurodegener. 2015 Mar 5;10:7. doi: 10.1186/s13024-015-0002-2. Mol Neurodegener. 2015. PMID: 25871877 Free PMC article. - Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4.
Masuoka N, Yoshimine C, Hori M, Tanaka M, Asada T, Abe K, Hisatsune T. Masuoka N, et al. Nutrients. 2019 Jul 17;11(7):1626. doi: 10.3390/nu11071626. Nutrients. 2019. PMID: 31319510 Free PMC article. Clinical Trial. - Hippocampal but Not Serum Cytokine Levels Are Altered by Traffic-Related Air Pollution in TgF344-AD and Wildtype Fischer 344 Rats in a Sex- and Age-Dependent Manner.
Patten KT, Valenzuela AE, Wallis C, Harvey DJ, Bein KJ, Wexler AS, Gorin FA, Lein PJ. Patten KT, et al. Front Cell Neurosci. 2022 Apr 22;16:861733. doi: 10.3389/fncel.2022.861733. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35530180 Free PMC article.
References
- Adams D, Hamilton T. The cell biology of macrophage activation. Ann Rev Immunol. 1984;2:283–318. - PubMed
- Avila E, Holdsworth G, Sasaki N, Jackson R, Harmony J. Apolipoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J Biol Chem. 1982;257:5900–5909. - PubMed
- Barger S, Harmon A. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. - PubMed
- Brown C, Wright E, Colton C, Sullivan P, Laskowitz D, Vitek M. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG005128/AG/NIA NIH HHS/United States
- R01 AG019780/AG/NIA NIH HHS/United States
- AG019780/AG/NIA NIH HHS/United States
- R01 AG023802/AG/NIA NIH HHS/United States
- AG023802-01/AG/NIA NIH HHS/United States
- P50 AG05128/AG/NIA NIH HHS/United States
- RF1 AG057895/AG/NIA NIH HHS/United States
- R01 AG019740/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous