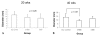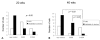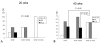Sulindac prevents esophageal adenocarcinomas induced by gastroduodenal reflux in rats - PubMed (original) (raw)
Sulindac prevents esophageal adenocarcinomas induced by gastroduodenal reflux in rats
Sung Wook Kim et al. Yonsei Med J. 2007.
Abstract
Purpose: It is known that cyclooxygenase (COX)-2 expression is increased in Barrett's esophagus and esophageal adenocarcinomas. We studied COX-2 expression and the effect sulindac has on the genesis of Barrett's esophagus and adenocarcinoma in rats undergoing esophagogastroduodenal anastomosis (EGDA).
Materials and methods: Fifty-one rats were divided into a control group (n=27), a 500 ppm sulindac-treated group (n=15) and 1000 ppm sulindac-treated group (n=9). Randomly selected rats were killed by diethyl ether inhalation at 20 and 40 weeks after surgery.
Results: At 40 weeks, rats treated with 1000 ppm sulindac showed narrower esophageal diameter and milder inflammation than the control rats. At 40 weeks, the incidence of Barrett's esophagus was similar between control and sulindac-treated groups, but the incidence of adenocarcinoma was significantly lower in the 1000 ppm sulindac-treated group than either the control or 500 ppm sulindac-treated groups. COX-2 was significantly increased in the lower esophagus of control rats killed at 40 weeks. Cyclin D1 expression was negligible in the sulindac- treated group compared with the control group.
Conclusion: We suggest that the chemopreventive effect of sulindac is related to decreased COX-2 and cyclin D1 expression, which may be influenced by reduced inflammation.
Figures
Fig. 1
The lower esophageal diameter of rats undergoing esophagogastroduodenal anastomosis for 20 weeks (A) and 40 weeks (B) according to sulindac treatment. Values are expressed as means ± SD. At 40 weeks, rats treated with 1000 ppm sulindac showed a diameter narrower than that of the control rats (p < 0.05).
Fig. 2
Lower esophageal inflammation in rats undergoing esophagogastroduodenal anastomosis for 20 weeks (A) and 40 weeks (B) according to sulindac treatment. At 40 weeks, rats treated with 1000 ppm sulindac showed milder inflammation than control rats or those treated with 500 ppm sulindac (p < 0.05).
Fig. 3
Histological findings (A, B) and immunohistochemical staining for BrdU (C, D) in the lower esophagus. Rats undergoing EGDA showed intestinal metaplasia including goblet cells above the esophagoduodenal junction (A) and adenocarcinoma characterized by abundant mucin secretion (B). BrdU-labeled columnar cells were located in the upper and lower portions of Barrett's esophagus (C). In duodenal mucosa, proliferating cells were mainly restricted to the isthmic portion (D). Magnification, × 200.
Fig. 4
Incidence of Barrett's esophagus and adenocarcinoma in rats undergoing esophagogastroduodenal anastomosis for 20 weeks (A) and 40 weeks (B) according to sulindac treatment. At 40 weeks, the adenocarcinoma incidence was significantly lower in rats treated with 1000 ppm sulindac than in the controls or 500 ppm sulindac-treated rats (p = 0.05).
Fig. 5
Western blot analysis of COX-2 and cyclin D1 expression in the lower esophagus of sham surgery rats and rats undergoing esophagogastroduodenal anastomosis for 20 weeks and 40 weeks according to sulindac treatment. Forty µg of protein was separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Immunoblots were probed with COX-2 and cyclin D1 antibodies. The bottom represents β-actin, which was used as a loading control.
Fig. 6
Western blot analysis of COX-2 and PCNA expression in the lower esophagus, stomach, and duodenum of rats undergoing esophagogastroduodenal anastomosis for 40 weeks. Forty µg of protein was separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Immunoblots were probed with COX-2 and PCNA antibodies. The bottom represents β-actin, which was used as a loading control.
Similar articles
- Thioproline inhibits development of esophageal adenocarcinoma induced by gastroduodenal reflux in rats.
Kumagai H, Mukaisho K, Sugihara H, Miwa K, Yamamoto G, Hattori T. Kumagai H, et al. Carcinogenesis. 2004 May;25(5):723-7. doi: 10.1093/carcin/bgh067. Epub 2004 Jan 30. Carcinogenesis. 2004. PMID: 14754873 - Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus.
Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Buttar NS, et al. Gastroenterology. 2002 Apr;122(4):1101-12. doi: 10.1053/gast.2002.32371. Gastroenterology. 2002. PMID: 11910360 - The sequential model of Barrett's esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens.
Sato T, Miwa K, Sahara H, Segawa M, Hattori T. Sato T, et al. Anticancer Res. 2002 Jan-Feb;22(1A):39-44. Anticancer Res. 2002. PMID: 12017320 - Duodenogastric reflux and foregut carcinogenesis.
Miwa K, Hattori T, Miyazaki I. Miwa K, et al. Cancer. 1995 Mar 15;75(6 Suppl):1426-32. doi: 10.1002/1097-0142(19950315)75:6+<1426::aid-cncr2820751506>3.0.co;2-#. Cancer. 1995. PMID: 7889469 Review. - [Reflux of duodenal or gastroduodenal contents induces esophageal carcinoma in rats].
Miwa K, Miyashita T, Hattori T. Miwa K, et al. Nihon Rinsho. 2004 Aug;62(8):1433-8. Nihon Rinsho. 2004. PMID: 15344531 Review. Japanese.
Cited by
- Barrett's esophagus: where do we stand?
Al Madi MA. Al Madi MA. Saudi J Gastroenterol. 2009 Jan;15(1):2-10. doi: 10.4103/1319-3767.45046. Saudi J Gastroenterol. 2009. PMID: 19568547 Free PMC article. - Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett's esophagus: a meta-analysis.
Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, Bruno M, Yu XJ, Zhou D, Zou XP. Zhang S, et al. Br J Cancer. 2014 Apr 29;110(9):2378-88. doi: 10.1038/bjc.2014.127. Epub 2014 Mar 20. Br J Cancer. 2014. PMID: 24651385 Free PMC article. - American Gastroenterological Association technical review on the management of Barrett's esophagus.
Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. Spechler SJ, et al. Gastroenterology. 2011 Mar;140(3):e18-52; quiz e13. doi: 10.1053/j.gastro.2011.01.031. Gastroenterology. 2011. PMID: 21376939 Free PMC article. Review. No abstract available.
References
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. - PubMed
- Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. - PubMed
- Barrett NR. The lower esophagus lined by columnar epithelium. Surgery. 1957;41:881–894. - PubMed
- Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett's esophagus. N Engl J Med. 1976;295:476–480. - PubMed
- Winters C, Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, 3rd, et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials