The ATPase SpoIIIE transports DNA across fused septal membranes during sporulation in Bacillus subtilis - PubMed (original) (raw)
The ATPase SpoIIIE transports DNA across fused septal membranes during sporulation in Bacillus subtilis
Briana M Burton et al. Cell. 2007.
Abstract
The FtsK/SpoIIIE family of ATP-dependent DNA transporters mediates proper chromosome segregation in dividing bacteria. In sporulating Bacillus subtilis cells, SpoIIIE translocates much of the circular chromosome from the mother cell into the forespore, but the molecular mechanism remains unclear. Using a new assay to monitor DNA transport, we demonstrate that the two arms of the chromosome are simultaneously pumped into the forespore. Up to 70 molecules of SpoIIIE are recruited to the site of DNA translocation and assemble into complexes that could contain 12 subunits. The fusion of the septal membranes during cytokinesis precedes DNA translocation and does not require SpoIIIE, as suggested by analysis of lipid dynamics, serial thin-section electron microscopy, and cell separation by protoplasting. These data support a model for DNA transport in which the transmembrane segments of FtsK/SpoIIIE form linked DNA-conducting channels across the two lipid bilayers of the septum.
Figures
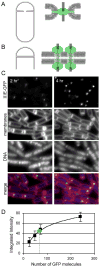
Figure 1
A large number of SpoIIIE molecules are recruited to the polar septum. (A) Models for SpoIIIE assembly at the septum. The cytoplasmic ATPase domains form an aqueous channel and the transmembrane segments anchor the complex in the invaginating septal annulus. The hexameric SpoIIIE ring accommodates a single double-stranded DNA (grey line). A single ring is depicted in the septal annulus, but the complex could also be formed by paired or stacked rings. (B) SpoIIIE forms separate DNA-conducting channels for each chromosome arm in the septal membranes. The ATPase domains and the transmembrane segments form these channels. (C) SpoIIIE-GFP was monitored by fluorescence microcopy in sporulating cells (strain BDR1626) grown at 30°C. The intensity of the GFP foci increased between hour two and hour four of sporulation. Membranes and DNA from the same fields were visualized using the fluorescent dyes FM4-64 and DAPI, respectively. Similar results were obtained from cells sporulated under more rapid conditions (Figure S4). (D) Calibration and quantitation of the number of GFP molecules. Sporulating cell harboring arrays of 12, 24, 36 and 120 tetO operators and saturating amounts of TetR-GFP were analyzed by fluorescence microscopy. The intensities of the flourescent foci (Figure S3) were plotted against the expected number of bound TetR-GFP molecules. TetR binds the tetO operator as a dimer. The green circle indicates the median integrated intensity of SpoIIIE-GFP foci.
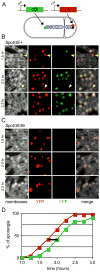
Figure 2
In vivo DNA transport assay. (A) Schematic of a sporulating cell used in this assay. For simplicity, only the chromosome transported by SpoIIIE is diagrammed. Red and green circles represent the location of the sigmaF-controlled reporter genes, yfp and cfp. The origin of the chromosome is marked with a black dot. (B) Sporulation (SpoIIIE+, strain BBB128) was induced by resuspension and YFP (pseudo-colored red) and CFP (pseudo-colored green) fluorescence were monitored over time. Examples of forespores with YFP fluorescence but not CFP fluorescence are indicated (yellow carets). (C) Sporulation (SpoIIIE36, strain BNS276) was induced by resuspension and YFP (pseudo-colored red) and CFP (pseudo-colored green) fluorescence were monitored over time. (D) Three fields of several hundred sporangia each were scored per time point (strain BBB128). The percent of sporangia expressing each reporter was plotted against time. The black bar denotes the average time (~20 min) between visualization of the origin and terminus reporters.
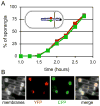
Figure 3
Simultaneous transport of the two chromosome arms into the forespore. (A) Analysis of fluorescence from a strain (BB162) that contains the cfp and yfp reporters on the left and right arms of the chromosome. The appearance of YFP and CFP in the forespore is similar over time. Three fields of several hundred sporangia each were scored for YFP and CFP fluorescence in the forespore. The percent of sporangia expressing each reporter was plotted against time. Inset shows a schematic of a cell and the location of the origin (black dot), the sigmaF-controlled yfp gene (red dot) and the sigmaF-controlled cfp gene (green dot). (B) Individual cell analysis demonstrates that essentially all forespores that contain one fluorescent marker contain the other. Thus, both arms are translocated simultaneously. A strain in which the positions of the two markers were swapped produced identical results (data not shown).
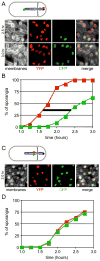
Figure 4
A slow-pumping mutant of SpoIIIE pumps both arms simultaneously. (A) Red and green dots represent the location of the sigmaF-controlled reporter genes, yfp and cfp. Sporulation in the SpoIIIE(D584A) mutant (strain BBB131) was induced by resuspension and YFP (pseudo-colored red) and CFP (pseudo-colored green) fluorescence were monitored over time. (B) Determination of the DNA transport rate for the slow-pumping mutant. Three fields of several hundred sporangia each were scored per time point. The percent of sporangia expressing each reporter was plotted against time. The black bar denotes the average time (~50 min) between visualization of the origin and terminus reporters. (C) Individual cell analysis from a strain (BBB213) harboring reporters on the left and right arms of the chromosome. The fluorescence micrographs demonstrate that nearly all forespores that contain one fluorescent marker contain the other. (D) In a population of sporulating cells, the appearance of YFP and CFP occurs at the same time. Three fields of several hundred sporangia each were scored for YFP and CFP fluorescence in the forespore. The percent of sporangia expressing each reporter was plotted against time.
Figure 5
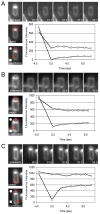
Membrane fusion at the sporulation septum occurs in the absence of SpoIIIE and DNA transport. Analysis of lipid dynamics using FM4-64 and fluorescence recovery after photobleaching (FRAP). Image series above the graphs show the photobleaching time course. Images to the side of the graphs indicate the region of photobleaching (top image, red circle) and the regions used for fluorescence quantitation (bottom image, red boxes). Graphs show forespore membrane (circles) and mother-cell membrane (squares) fluorescence over the course of the experiment. (A) Photobleaching of the forespore membranes in a spoIIIE36 mutant (strain BDR1050). (B) Photobleaching of the mother cell membranes in a spoIIIE null mutant (strain BDR1832). (C) Photobleaching of the forespore membranes in a spoIIIE null mutant.
Figure 6
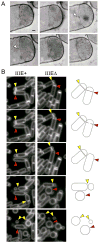
The mother and forespore compartments are separated by two complete septal membranes at the time of DNA transport. (A) Serial thin section electron micrographs of a sporulating cell expressing a functional spoIIIE-gfp fusion (strain BDR1626), immunostained with gold conjugated anti-GFP antibodies. The gold particles (carets in two middle sections) identify SpoIIIE-GFP. Scale bar is 100 nm. Larger versions of these images can be found in Supplemental Figure S9A. In the cell shown, gold particle labeling is only observed on the mother cell side of the seputm. Gold particle labeling of SpoIIIE-GFP can be observed on both sides of the septum (See Figure S9B). (B) Time-lapse images of sporulating cells treated with lysozyme. The protoplasted strains were wild type spoIIIE (SpoIIIE+) (BTD1559) or a spoIIIE null mutant (BBB339). Both strains contained a spoIIQ mutation to prevent protoplast engulfment (Broder and Pogliano, 2006). The time-lapse movies can be found in Supplemental Material (Movie M1A and M1B). Representative cells in which the polar septa have fused are indicated (yellow carets). Septa that have not yet fused and reverse upon lysozyme-treatment are also highlighted (red carets).
Figure 7
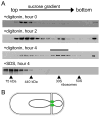
SpoIIIE assembles into large complexes. (A) Analysis of SpoIIIE-GFP complexes from sporulating cells. Samples were harvested at the initiation of sporulation (hour zero), and after two and fours hours. Detergent-solubilized extracts from membrane fractions were loaded onto linear (10–40%) sucrose gradients. Fractions were analyzed by immunoblot with anti-GFP antibodies. The high molecular weight peak of SpoIIIE in the hour 4 gradient is indicated (bar). Molecular weight standards of 75 and 440kDa, as well as 30S and 50S E. coli ribosomes were analyzed in a parallel gradient. The peak fraction for each size marker is indicated with a caret. Differences in intensities between blots are due to variation in lysis efficiency and protein transfer. (B) Schematic diagram of two DNA-conducting channels at the sporulation septum.
Similar articles
- Evidence that the SpoIIIE DNA translocase participates in membrane fusion during cytokinesis and engulfment.
Liu NJ, Dutton RJ, Pogliano K. Liu NJ, et al. Mol Microbiol. 2006 Feb;59(4):1097-113. doi: 10.1111/j.1365-2958.2005.05004.x. Mol Microbiol. 2006. PMID: 16430687 Free PMC article. - Dynamic SpoIIIE assembly mediates septal membrane fission during Bacillus subtilis sporulation.
Fleming TC, Shin JY, Lee SH, Becker E, Huang KC, Bustamante C, Pogliano K. Fleming TC, et al. Genes Dev. 2010 Jun 1;24(11):1160-72. doi: 10.1101/gad.1925210. Genes Dev. 2010. PMID: 20516200 Free PMC article. - FtsK and SpoIIIE, coordinators of chromosome segregation and envelope remodeling in bacteria.
Chan H, Mohamed AMT, Grainge I, Rodrigues CDA. Chan H, et al. Trends Microbiol. 2022 May;30(5):480-494. doi: 10.1016/j.tim.2021.10.002. Epub 2021 Oct 30. Trends Microbiol. 2022. PMID: 34728126 Review. - Role of cell-specific SpoIIIE assembly in polarity of DNA transfer.
Sharp MD, Pogliano K. Sharp MD, et al. Science. 2002 Jan 4;295(5552):137-9. doi: 10.1126/science.1066274. Science. 2002. PMID: 11778051 Free PMC article. - The FtsK family of DNA translocases finds the ends of circles.
Crozat E, Rousseau P, Fournes F, Cornet F. Crozat E, et al. J Mol Microbiol Biotechnol. 2014;24(5-6):396-408. doi: 10.1159/000369213. Epub 2015 Feb 17. J Mol Microbiol Biotechnol. 2014. PMID: 25732341 Review.
Cited by
- A septal chromosome segregator protein evolved into a conjugative DNA-translocator protein.
Sepulveda E, Vogelmann J, Muth G. Sepulveda E, et al. Mob Genet Elements. 2011 Sep;1(3):225-229. doi: 10.4161/mge.1.3.18066. Epub 2011 Sep 1. Mob Genet Elements. 2011. PMID: 22479692 Free PMC article. - Xer Site Specific Recombination: Double and Single Recombinase Systems.
Castillo F, Benmohamed A, Szatmari G. Castillo F, et al. Front Microbiol. 2017 Mar 20;8:453. doi: 10.3389/fmicb.2017.00453. eCollection 2017. Front Microbiol. 2017. PMID: 28373867 Free PMC article. Review. - Contribution of SMC (structural maintenance of chromosomes) and SpoIIIE to chromosome segregation in Staphylococci.
Yu W, Herbert S, Graumann PL, Götz F. Yu W, et al. J Bacteriol. 2010 Aug;192(15):4067-73. doi: 10.1128/JB.00010-10. Epub 2010 Jun 4. J Bacteriol. 2010. PMID: 20525833 Free PMC article. - Hierarchical evolution of the bacterial sporulation network.
de Hoon MJ, Eichenberger P, Vitkup D. de Hoon MJ, et al. Curr Biol. 2010 Sep 14;20(17):R735-45. doi: 10.1016/j.cub.2010.06.031. Curr Biol. 2010. PMID: 20833318 Free PMC article. Review. - Mutations altering a structurally conserved loop-helix-loop region of a viral packaging motor change DNA translocation velocity and processivity.
Tsay JM, Sippy J, DelToro D, Andrews BT, Draper B, Rao V, Catalano CE, Feiss M, Smith DE. Tsay JM, et al. J Biol Chem. 2010 Jul 30;285(31):24282-9. doi: 10.1074/jbc.M110.129395. Epub 2010 Jun 4. J Biol Chem. 2010. PMID: 20525695 Free PMC article.
References
- Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt D. FtsK Is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell. 2002;108:195–205. - PubMed
- Bartosik AA, Jagura-Burdzy G. Bacterial chromosome segregation. Acta Biochim Pol. 2005;52:1–34. - PubMed
- Bath J, Wu LJ, Errington J, Wang JC. Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science. 2000;290:995–97. - PubMed
- Ben-Yehuda S, Rudner DZ, Losick R. Assembly of the SpoIIIE DNA translocase depends on chromosome trapping in Bacillus subtilis. Curr Biol. 2003;13:2196–2200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases