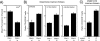The proteasome immunosubunit multicatalytic endopeptidase complex-like 1 is a T-cell-intrinsic factor influencing homeostatic expansion - PubMed (original) (raw)
The proteasome immunosubunit multicatalytic endopeptidase complex-like 1 is a T-cell-intrinsic factor influencing homeostatic expansion
Dietmar M W Zaiss et al. Infect Immun. 2008 Mar.
Abstract
Homeostatic regulatory mechanisms maintain the constant ratios between different lymphocyte subsets in the secondary lymphoid organs. How this dynamic equilibrium is achieved, in particular following the clonal expansion and subsequent contraction of different cells after infection, remains poorly understood. Expression of the proteasome immunosubunits has been shown to influence not only major histocompatibility complex class I (MHC-I) antigen processing and thereby T-cell responses, but also the CD4/CD8 T-cell ratios in lymphoid organs. We examined the relationships between these different immunosubunit-mediated effects in mice of various proteasome subunit compositions during infection with Listeria monocytogenes. Mice that lacked the immunosubunit multicatalytic endopeptidase complex-like 1 (MECL-1) maintained enhanced CD4/CD8 T-cell ratios during infection, while MHC-I surface levels resembled those in wild-type (wt) mice. LMP7 gene-deficient mice, on the other hand, showed reduced MHC-I expression, while their splenic CD4/CD8 ratios were similar to those in wt mice. Remarkably, analysis of bone marrow-chimeric immunosubunit gene-deficient mice, reconstituted with a mixture of wt and LMP7- plus MECL-1-deficient bone marrow, revealed that the LMP7- plus MECL-1-deficient T-cell population maintained a higher CD4/CD8 T-cell ratio than the wt T-cell population before, during, and after infection and T-cell memory formation. Since in these mice the immunosubunit-positive and immunosubunit-negative T-cell populations were selected in the same thymus and expanded in the same lymphoid environments, our findings indicate that MECL-1 influences the homeostatic equilibrium between T-cell subsets, not through indirect extracellular signals, such as MHC-I expression or the cytokine milieu, but through direct effects on T-cell-intrinsic processes.
Figures
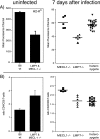
FIG. 1.
Effects of LMP7 and MECL-1 on MHC-I cell surface expression (A) and CD4/CD8 ratios (B) in uninfected and _L. monocytogenes_-infected mice. Splenocytes of age-matched B6 wt, MECL-1−/−, LMP7−/−, and LMP7−/− plus MECL-1−/− mice were stained with anti-CD19-APC, biotin-conjugated anti-H-2Kb, and SAV-PE (uninfected mice; n = 4); anti-CD19-PE, biotin-conjugated anti-H-2Kb, and SAV-APC (infected mice) (A); or anti-CD4-FITC and anti-CD8α-APC antibodies (B) and analyzed by flow cytometry. Mean fluorescence channels of the CD19+ populations (A) and CD4/CD8 T-cell ratios (means plus standard deviations; n = 5 or 6) (B) are depicted.
FIG. 2.
Effects of LMP7 and MECL-1 in _Listeria_-infected mice. F2 offspring of B6 wt × LMP7−/− plus MECL-1−/− mice were infected with 5 × 103 rLM-E1 cells intravenously. The spleens were collected at day 7 after infection and analyzed for the presence of LMP7 and/or MECL-1 by immunoblot analysis, using specific rabbit antisera (x axis). (A) Total numbers of splenocytes per spleen (means plus standard deviations). (B and C) Cells were stained with anti-CD8α-APC and anti-CD4-FITC to determine the relative frequencies of CD8 and CD4 T cells, respectively. (D) Cells were incubated with 500 nM synthetic E1B192-200 for 6 h in the presence of monensin, and the frequencies of E1B192-200-specific CD8 T cells were determined by staining for CD8 (anti-CD8α-FITC) and intracellular IFN-γ (IFN-γ-PE). Background, detected in samples incubated without peptide, was subtracted.
FIG. 3.
MHC-I cell surface levels and CD4/CD8 T-cell ratios of LMP7pos plus MECL-1pos and LMP7neg plus MECL-1neg T-cell subsets in mixed BM-chimeric mice. Lethally irradiated LMP7−/− plus MECL-1−/− mice were reconstituted with a mixture of BM from B6.SJL (wt; CD45.1pos) and LMP7−/− plus MECL-1−/− (ko; CD45.2pos) mice and 28 days later were infected with rLM-E1. (A) Splenocytes of mixed BM-chimeric mice were stained at day 28 after BM injection with fluorochrome-conjugated anti-CD45.1, anti-CD45.2, anti-CD19, and biotin-conjugated anti-H-2Kb and SAV-APC to determine the H-2Kb expression levels on the CD45.1 and CD45.2 B-cell subsets. Mean fluorescence channels are depicted (means plus standard deviations [SD]; n = 2). (B) Relative frequencies of CD45.1pos and CD45.2pos CD4 and CD8 T cells in the spleens of mixed BM-chimeric mice were determined at day 28 after BM injection and at days 7 and 46 following Listeria infection by staining with fluorochrome-conjugated anti-CD45.1, anti-CD45.2, anti-TCRβ, anti-CD4, and anti-CD8α antibodies. CD4/CD8 T-cell ratios are depicted (means plus SD; n = 2 to 5). The data are representative of two independent experiments. (C) Relative frequencies of CD4 and CD8 T cells in the spleens of CD45.1pos wt recipients reconstituted with CD45.2pos LMP7−/− plus MECL-1−/− BM and LMP7−/− plus MECL-1−/− recipients reconstituted with wt BM (means plus SD; n = 4).
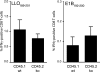
FIG. 4.
T-cell responses in rLM-E1-infected mixed BM-chimeric mice. Mixed BM chimeras were infected with rLM-E1, and the relative frequencies of CD45.1pos and CD45.2pos T cells in the spleens specific for the rLM-E1-derived CD8 T-cell epitope E1B192-200 and CD4 T-cell epitope LLO189-201, were determined by intracellular IFN-γ staining (means plus standard deviations; n = 5). Background, detected in samples incubated without peptide, was subtracted.
Similar articles
- T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens.
Caudill CM, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. Caudill CM, et al. J Immunol. 2006 Apr 1;176(7):4075-82. doi: 10.4049/jimmunol.176.7.4075. J Immunol. 2006. PMID: 16547243 - An altered T cell repertoire in MECL-1-deficient mice.
Basler M, Moebius J, Elenich L, Groettrup M, Monaco JJ. Basler M, et al. J Immunol. 2006 Jun 1;176(11):6665-72. doi: 10.4049/jimmunol.176.11.6665. J Immunol. 2006. PMID: 16709825 - Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice.
Moebius J, van den Broek M, Groettrup M, Basler M. Moebius J, et al. Eur J Immunol. 2010 Dec;40(12):3439-49. doi: 10.1002/eji.201040620. Epub 2010 Nov 11. Eur J Immunol. 2010. PMID: 21108466 - [Proteasome and antigen presentation].
Tanaka K. Tanaka K. Seikagaku. 1993 Apr;65(4):286-92. Seikagaku. 1993. PMID: 8315310 Review. Japanese. No abstract available. - To DRiP or not to DRiP: generating peptide ligands for MHC class I molecules from biosynthesized proteins.
Yewdell J. Yewdell J. Mol Immunol. 2002 Oct;39(3-4):139-46. doi: 10.1016/s0161-5890(02)00097-4. Mol Immunol. 2002. PMID: 12200046 Review. No abstract available.
Cited by
- The dichotomous role of immunoproteasome in cancer: Friend or foe?
Chen B, Zhu H, Yang B, Cao J. Chen B, et al. Acta Pharm Sin B. 2023 May;13(5):1976-1989. doi: 10.1016/j.apsb.2022.11.005. Epub 2022 Nov 5. Acta Pharm Sin B. 2023. PMID: 37250147 Free PMC article. Review. - The Immunoproteasome Subunits LMP2, LMP7 and MECL-1 Are Crucial Along the Induction of Cerebral Toxoplasmosis.
French T, Israel N, Düsedau HP, Tersteegen A, Steffen J, Cammann C, Topfstedt E, Dieterich D, Schüler T, Seifert U, Dunay IR. French T, et al. Front Immunol. 2021 Apr 21;12:619465. doi: 10.3389/fimmu.2021.619465. eCollection 2021. Front Immunol. 2021. PMID: 33968021 Free PMC article. - Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses.
Hensley SE, Zanker D, Dolan BP, David A, Hickman HD, Embry AC, Skon CN, Grebe KM, Griffin TA, Chen W, Bennink JR, Yewdell JW. Hensley SE, et al. J Immunol. 2010 Apr 15;184(8):4115-22. doi: 10.4049/jimmunol.0903003. Epub 2010 Mar 12. J Immunol. 2010. PMID: 20228196 Free PMC article. - At the Cutting Edge against Cancer: A Perspective on Immunoproteasome and Immune Checkpoints Modulation as a Potential Therapeutic Intervention.
Tundo GR, Sbardella D, Oddone F, Kudriaeva AA, Lacal PM, Belogurov AA Jr, Graziani G, Marini S. Tundo GR, et al. Cancers (Basel). 2021 Sep 28;13(19):4852. doi: 10.3390/cancers13194852. Cancers (Basel). 2021. PMID: 34638337 Free PMC article. Review. - Proteostasis Dysfunction in Aged Mammalian Cells. The Stressful Role of Inflammation.
Ruano D. Ruano D. Front Mol Biosci. 2021 Jun 17;8:658742. doi: 10.3389/fmolb.2021.658742. eCollection 2021. Front Mol Biosci. 2021. PMID: 34222330 Free PMC article. Review.
References
- Basler, M., J. Moebius, L. Elenich, M. Groettrup, and J. J. Monaco. 2006. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 1766665-6672. - PubMed
- Caudill, C. M., K. Jayarapu, L. Elenich, J. J. Monaco, R. A. Colbert, and T. A. Griffin. 2006. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 1764075-4082. - PubMed
- De, M., K. Jayarapu, L. Elenich, J. J. Monaco, R. A. Colbert, and T. A. Griffin. 2003. Beta 2 subunit propeptides influence cooperative proteasome assembly. J. Biol. Chem. 2786153-6159. - PubMed
- Deol, P., D. M. Zaiss, J. J. Monaco, and A. J. Sijts. 2007. Rates of processing determine the immunogenicity of immunoproteasome-generated epitopes. J. Immunol. 1787557-7562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous