Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal - PubMed (original) (raw)
Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal
Francesca Balordi et al. J Neurosci. 2007.
Abstract
The Smoothened gene is necessary for cells to transduce hedgehog signaling. Although we and others have previously shown that embryonic removal of Smoothened in the neural tube results in a loss of stem cells from the postnatal subventricular zone, it was unclear whether this reflected a requirement for hedgehog signaling in the establishment or maintenance of the adult niche. Here, we have examined the consequences of conditional removal of Smoothened gene function within the subventricular zone of the adult neural stem cell niche. We observe that both proliferation and neurogenesis are compromised when hedgehog signaling is removed from subventricular zone stem cells. Moreover, even after a 10 month survival period, the stem cell niche fails to recover. It has been reported that the adult subventricular zone quickly rebounds from an antimitotic insult by increasing proliferation and replenishing the niche. During this recovery, it has been reported that hedgehog signaling appears to be upregulated. When mice in which hedgehog signaling in the subventricular zone has been strongly attenuated are given a similar antimitotic treatment, recovery is limited to the reduced level of proliferation and neurogenesis observed before the mitotic insult. Furthermore, the limited recovery that is observed appears to be largely restricted to the minority of neural stem cells that escape the conditional inactivation of Smoothened gene function. These results demonstrate that ongoing hedgehog signaling is required to maintain adult neural stem cells and that their ability to self-renew is limited.
Figures
Figure 1.
Generation of the Nestin CreERT2 transgenic mouse line. a, Scheme of the transgenic construct used to make the line. b–e, Coronal views of the embryonic (a, c) and adult (d, e) SVZ showing the pattern of expression of the transgene by Cre in situ hybridization (b, d), compared with the expression pattern of Nestin (c, e). Scale bars: 200 μm.
Figure 2.
Tamoxifen can efficiently induce Cre recombinase activity in the SVZ and in the SGL in adulthood. a–f, Coronal views of the embryonic (a, b) and adult (c, d) SVZ and the adult SGL (e, f) showing β-Gal staining representing the expression of LacZ, in Nestin CreER/+; Rosa stop LacZ/+ mice not treated with tamoxifen (a, c, e), and in Nestin CreER/+; Rosa stop LacZ/+ mice treated with tamoxifen (b, d, f). For embryonic analysis, tamoxifen was administrated once at E12.5 and analysis was done 1 d later (b). For adult analysis, tamoxifen was administrated at P60 for 3 consecutive days and analysis was done 1 d later (d, f). Scale bars: a–d, 200 μm; e, f, 100 μm; a'–d“, 50 μm.
Figure 3.
Our Nestin CreERT2 transgenic line can be used to visualize the continual production of new neurons, throughout adult life, in the germinal centers. a–p, Coronal (a–d, i–l) and sagittal (e–h, m–p) views of the adult SVZ (a–d), RMS (e–h), OB (i–l), and SGL (m–p) showing β-Gal staining representing the expression of LacZ, in Nestin CreER/+; Rosa stop LacZ/+ mice treated once with tamoxifen at P120 and analyzed 3 d later (a, e, i, m), 1 week later (b, f, j, n), 1 month later (c, g, k, o), and 2 months later (d, h, i, p). Scale bars: (in d, l, p) a–d, i–p, 200 μm; (in h) e–h, 400 μm.
Figure 4.
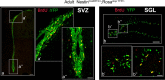
Cells undergoing Cre-induced recombination are proliferating cells, in the adult SVZ and SGL. a, b, Coronal views of the adult SVZ (a) and SGL (b) showing double labeling for BrdU (in red) and YFP (in green, detected by GFP antibody) in tamoxifen-treated Nestin CreER/+; Rosa stop YFP/+ mice. Scale bars: a, b, 100 μm; a'–b“, 25 μm.
Figure 5.
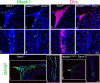
Loss of Hh signaling in tamoxifen-treated Nestin CreER; Smo c/c mice in the adult SVZ. a, Diagram of the tamoxifen treatment the Nestin CreER/+; Smo c/c mice (ko) and Smo c/c mice (controls) undergo, combined with the BrdU treatment. b, c, Coronal views of the adult SVZ showing β-Gal staining representing the expression pattern of the readout of Hh pathway, Gli1, in tamoxifen-treated Smo c/c; Gli1 LacZ/+ mice (b, b') and tamoxifen-treated Nestin CreER/+; Smo c/c; Gli1 LacZ/+ mice (c, c'). d, A quantification of the reduction in Gli1 expression in the SVZ. e, Results of PCR genotyping performed on individual neurospheres obtained from tamoxifen-treated Nestin CreER/+; Smo c/c mice. Note that n/n means that both events of recombination occurred; c/n means that only one event of recombination occurred; c/c indicate that no recombination occurred in the Smo conditional alleles. f, g, Coronal views of the adult SVZ showing BrdU-incorporating cells in tamoxifen-treated Smo c/c mice (f–f“) and in tamoxifen-treated Nestin CreER/+; Smo c/c mice (g–g”) soon after BrdU injections. h, Quantification of the data shown in f and g. i, Quantification of the numbers of neurospheres generated from the SVZ of tamoxifen-treated Nestin CreER/+; Smo c/c and controls. Scale bars: b, c, 200 μm; b', c', 50 μm; f, g, 150 μm; f', f“, g', g”, 30 μm. Error bars represent SD. Statistics were performed by t test, p < 0.05. In d, h, i, n = 3 animals each. In e, n(NSPHs) = 925, 4 animals.
Figure 6.
In tamoxifen-treated Nestin CreER/+; Smo c/c mice compared with controls, all cells types in the SVZ lineage are affected. a–d, Coronal views of two areas of the SVZ (the dorsal and the ventral parts) showing Mash1 immunostaining in tamoxifen-treated Smo c/c mice (a, b) and in tamoxifen-treated Nestin CreER/+; Smo c/c mice (c, d). Note that there is a 75% reduction in Mash1 expression. e–h, Coronal views of two areas of the SVZ showing Dcx immunostaining in tamoxifen-treated Smo c/c mice (e, f) and in tamoxifen-treated Nestin CreER/+; Smo c/c mice (g, h). Note that there is a 70% reduction in Dcx expression. i–l, Coronal views of two areas of the SVZ showing GFAP immunostaining in tamoxifen-treated Smo c/c mice (i, j) and in tamoxifen-treated Nestin CreER/+; Smo c/c mice (k, l). Note that there is an 80% reduction in GFAP expression. Scale bars: (in h) a–j, 30 μm; (in l) i–l, 30 μm.
Figure 7.
Partial replenishment of the SVZ stem cell niche in tamoxifen-treated Nestin CreER/+; Smo c/c mice after AraC injury in vivo. a–d, Coronal views of the SVZ showing BrdU-incorporating cells soon after the removal of the pump (t = 0; a, b) and 1 week after pump removal (t = 7; c, d) in AraC-treated controls (tamoxifen-treated Smo c/c mice; a, c) and AraC-treated ko animals (tamoxifen-treated Nestin CreER/+; Smo c/c mice; b, d). Scale bars: a–d, 100 μm; c', c', d', d', 30 μm.
Figure 8.
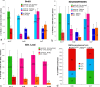
The partial replenishment of the SVZ stem cell niche in knock-out (ko) mice after AraC treatment is mainly performed by few spared Smo c/c and Smo c/n B cells. a, Quantification of the general level of proliferation in the SVZ in controls (tamoxifen-treated Smo c/c) and ko (tamoxifen-treated Nestin CreER/+; Smo c/c) animals before the implantation of the pump (t = −7), in saline-treated controls and ko, and in AraC-treated controls and ko, soon after the removal of the pump (t = 0) and 1 week after pump removal (t = 7). b, Quantification of the ability to generate neurospheres in controls (tamoxifen-treated Smo c/c) and ko (tamoxifen-treated Nestin CreER/+; Smo c/c) animals before the implantation of the pump (t = −7) in saline-treated controls and ko, and in AraC-treated controls and ko, soon after the removal of the pump (t = 0) and 1 week after pump removal (t = 7). c, Quantification of the numbers of β-Gal + cells, representing the expression pattern of Gli1, in controls (tamoxifen-treated Smo c/c;Gli1 LacZ/+) and ko (tamoxifen-treated Nestin CreER/+;Smo c/c;Gli1 LacZ/+) animals before the implantation of the pump (t = −7), in AraC-treated controls and AraC-treated ko animals, soon after the removal of the pump (t = 0), one week after pump removal (t = 7) and 1 month after pump removal (t = 30). d, Results of PCR genotyping performed on individual neurospheres obtained from tamoxifen-treated Nestin CreER/+; Smo c/c mice that received AraC treatment, at t = 0 and t = 7. Note that n/n means that both events of recombination occurred; c/n means that only one event of recombination occurred; c/c indicate that no recombination occurred within the Smo conditional alleles. In all panels t = −7 means without pump, t = 0 means soon after pump removal and t = 7 means 1 week after pump removal. Error bars represent SD. Statistics were performed by t test, p < 0.05. In a–c, n = 3 animals each; in d, t = 0, n(NSPHs) = 480, 3 animals; t = 7, n(NSPHs) = 405, 3 animals.
Figure 9.
The self-renewal property of SVZ stem cells in vivo is affected by the loss of Hh signaling. In tamoxifen-treated Nestin CreER/+; Smo c/c mice, ∼70% of SVZ progenitors are Smo n/n, whereas 30% are not fully knocked out (Smo c/c and Smo c/n), according to the NSPHs PCR genotyping shown in Figure 5_e_. After the AraC injury, the Smo n/n B cells are not able to self-renew, whereas the spared wt B cells (Smo c/c and Smo c/n) can self-renew but are not able to make up for the entire SVZ, suggesting that they might be competent to regenerate only a small local portion of SVZ. Adapted from Doetsch et al. (1999) with permission from Alvarez-Buylla.
Similar articles
- Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state.
Basak O, Giachino C, Fiorini E, Macdonald HR, Taylor V. Basak O, et al. J Neurosci. 2012 Apr 18;32(16):5654-66. doi: 10.1523/JNEUROSCI.0455-12.2012. J Neurosci. 2012. PMID: 22514327 Free PMC article. - Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone.
Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Charytoniuk D, et al. Eur J Neurosci. 2002 Dec;16(12):2351-7. doi: 10.1046/j.1460-9568.2002.02412.x. Eur J Neurosci. 2002. PMID: 12492430 - The subventricular zone: new molecular and cellular developments.
Conover JC, Allen RL. Conover JC, et al. Cell Mol Life Sci. 2002 Dec;59(12):2128-35. doi: 10.1007/s000180200012. Cell Mol Life Sci. 2002. PMID: 12568338 Free PMC article. Review. - Recovery of Brain in Chick Embryos by Growing Second Heart and Brain.
Fioranelli M, Sepehri A, Roccia MG, Linda C, Rossi C, Dawodo A, Vojvodic P, Lotti J, Barygina V, Vojvodic A, Wollina U, Tirant M, Thuong NV, Lotti T. Fioranelli M, et al. Open Access Maced J Med Sci. 2019 Aug 30;7(18):3085-3089. doi: 10.3889/oamjms.2019.777. eCollection 2019 Sep 30. Open Access Maced J Med Sci. 2019. PMID: 31850128 Free PMC article. Retracted. Review.
Cited by
- The Principle of Cortical Development and Evolution.
Yang Z. Yang Z. Neurosci Bull. 2024 Jul 18. doi: 10.1007/s12264-024-01259-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39023844 Review. - Developmental trajectories of GABAergic cortical interneurons are sequentially modulated by dynamic FoxG1 expression levels.
Miyoshi G, Ueta Y, Yagasaki Y, Kishi Y, Fishell G, Machold RP, Miyata M. Miyoshi G, et al. Proc Natl Acad Sci U S A. 2024 Apr 16;121(16):e2317783121. doi: 10.1073/pnas.2317783121. Epub 2024 Apr 8. Proc Natl Acad Sci U S A. 2024. PMID: 38588430 Free PMC article. - Single-Cell Integration of BMD GWAS Results Prioritize Candidate Genes Influencing Age-Related Bone Loss.
Doolittle ML, Khosla S, Saul D. Doolittle ML, et al. JBMR Plus. 2023 Jul 7;7(10):e10795. doi: 10.1002/jbm4.10795. eCollection 2023 Oct. JBMR Plus. 2023. PMID: 37808401 Free PMC article. - The Past, Present, and Future of Genetically Engineered Mouse Models for Skeletal Biology.
Michalski MN, Williams BO. Michalski MN, et al. Biomolecules. 2023 Aug 26;13(9):1311. doi: 10.3390/biom13091311. Biomolecules. 2023. PMID: 37759711 Free PMC article. Review. - Endogenous Neural Stem Cell Mediated Oligodendrogenesis in the Adult Mammalian Brain.
Radecki DZ, Samanta J. Radecki DZ, et al. Cells. 2022 Jul 2;11(13):2101. doi: 10.3390/cells11132101. Cells. 2022. PMID: 35805185 Free PMC article. Review.
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Aihara M, Sugawara K, Torii S, Hosaka M, Kurihara H, Saito N, Takeuchi T. Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest. 2004;84:1581–1592. - PubMed
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases