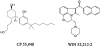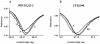Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling - PubMed (original) (raw)
Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling
Teodora Georgieva et al. Eur J Pharmacol. 2008.
Abstract
Cannabinoid drugs differ in their rank order of potency to produce analgesia versus other central nervous system effects. We propose that these differences are due to unique agonist-bound cannabinoid CB1 receptor conformations that exhibit different affinities for individual subsets of intracellular signal transduction pathways. In order to test this hypothesis, we have used plasmon-waveguide resonance (PWR) spectroscopy, a sensitive method that can provide direct information about ligand-protein and protein-protein interactions, and can detect conformational changes in lipid-embedded proteins. A recombinant epitope-tagged human cannabinoid CB1 receptor was expressed in insect Sf9 cells, solubilized and purified using two-step affinity chromatography. The purified receptor was incorporated into a lipid bilayer on the surface of the PWR resonator. PWR spectroscopy demonstrated that cannabinoid agonists exhibit high affinity (KD=0.2+/-0.03 nM and 2+/-0.4 nM for CP 55,940 and WIN 55,212-2, respectively) for the purified epitope tagged hCB(1) receptor. Interestingly however, these structurally different cannabinoid agonists shifted the PWR spectra in opposite directions, indicating that CP 55,940 and WIN 55,212-2 binding leads to different hCB1 receptor conformations. Furthermore, PWR experiments also indicated that these CP 55,940-and WIN 55,212-bound hCB1 receptor conformations exhibit slightly different affinities to an inhibitory G protein heterotrimer, Gi1 (KD=27+/-8 nM and KD=10.7+/-4.7 nM, respectively), whereas they strikingly differ in their ability to activate this G protein type.
Figures
Fig. 1
Structure of bicyclic (CP-55,940) and aminoalkylindole (WIN 55,212-2) cannabinoid receptor agonists.
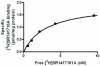
Fig. 2. Saturation isotherm for [3H] SR141716A specific binding to human cannabinoid CB1- myc-His6 receptor /Sf9 cell membranes
Infection of Sf9 cells with the cannabinoid CB1- myc-His6 receptor -containing bacmid led to the expression of 2.4 ± 0.3 pmol/mg protein (mean ± S.E.M., n=4) [3H] SR141716A specific binding sites, with a KD value of 2.9 ± 0.8 nM. The figure is a representative of measurements (done in duplicates) performed for four independent infections.
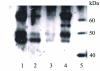
Fig. 3. Enrichment of the fully glycosylated form (64 kDa) of human cannabinoid CB1- myc-His6 receptor by lectin affinity chromatography and gradient elution
The figure shows a representative Western blot probed with an antibody raised against the N-terminus of the human cannbinoid CB1 receptor. 1. solubilized Sf9/ human cannabinoid CB1- myc-His6 receptor membrane preparation. Glycosylated proteins were eluted from the WGA column with increasing concentrations of 2. 0.125 M, 3. 0.25 M and 4. 0.5 M N-acetylglucosamine; 5. molecular weight markers.
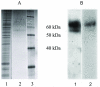
Fig. 4. Purification of the WGA column eluate using metal affinity chromatography
A. Silver stained SDS-polyacrylamide gel: 1. solubilized membrane preparation, 2. partially purified human cannabinoid CB1- myc-His6 receptor, 3. MW markers. B. Western blot of the final, partially purified human cannabinoid CB1- myc-His6 receptor preparation probed with: 1. an anti-human cannabinoid CB1 antibody, 2. an anti- myc antibody. 1 μg protein has been loaded on each lane.
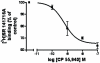
Fig. 5. Inhibition of specific [3H]SR 141716A binding to the partially purified human cannabinoid 1- myc-His6 receptor receptor by a cannabinoid 1 selective agonist, CP 55,940
The data represent the mean value of two independent experiments done in duplicates. IC50 = 0.6 ± 0.2 nM (mean ± range).
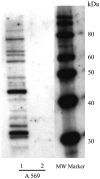
Fig. 6. The human cannabinoid 1- myc-His6 receptor does not co-purify with insect G proteins from infected Sf9 cells
A representative Western blot of the 1. solubilized and 2. partially purified human cannabinoid CB1- myc-His6 receptor preparations, probed with a nonselective anti-G protein α-subunit antibody (A569). Equal amounts of 2 μg total protein have been loaded on lanes 1 and 2.
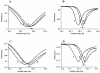
Fig. 7. Two structurally different cannabinoid agonists, CP 55,940 (A and B) and WIN 55,212-2 (C and D) shift the PWR angle in different directions
PWR spectra for the: 1. empty PC/POPG lipid bilayer; 2. the proteolipid bilayer after incorporation of the partially purified human cannabinoid CB1- myc-His6 receptor; and 3. human cannabinoid 1- myc-His6 receptor -containing lipid bilayer after the addition of saturating concentrations of CP 55,940 (3 nM) or WIN 55,212-2 (10 nM). The spectra were obtained with laser light polarized in _p_- (A and C) or _s_- (B and D) directions. Arrows indicate the directions of the PWR angle shifts upon agonist addition. The spectra are representative from three independent experiments.
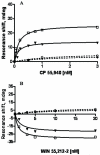
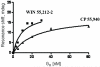
Fig. 8. Agonist dose-response curves for CP 55,940 (A) and WIN 55,212-2 (B)
PWR angle shifts (deg) were measured after addition of increasing concentrations of the appropriate agonist into the PWR chamber, containing either an empty PC/POPG lipid bilayer (dashed lines) or the human cannabinoid CB1– myc-His6 receptor -containing proteolipid system (solid lines). PWR spectral shifts (representative experiments from three independent experiments are shown) were obtained using _p_- (open symbols) or _s_- (solid symbols) polarized laser light. Lines through the data points were obtained by a hyperbolic fit using Graph Pad Prism; KD values are given in the text.
Fig. 9. Dose-PWR response curves for Gi1 protein binding to agonist-bound human cannabinoid CB1–myc-His6 receptor conformations
PWR spectra were measured after addition of increasing concentrations of GDP-bound Gi1 heterotrimer (1:1 mixture of recombinant myristoylated Giα1 and purified βγ-subunits from bovine brain) to the aqueous compartment of the PWR resonator containing WIN 55,212-2 (■) or CP 55,940 (▲)-bound epitope tagged CB1 receptors. PWR spectra were obtained with _p_-polarized light; similar dose-response curves were obtained using _s_-polarization (not shown). Lines through the data points were obtained by a hyperbolic fit; KD values are given in the text. The spectra are representative from three independent experiments.
Fig. 10. The effect of saturating concentrations of GTPγS on the PWR spectra of cannabinoid agonist/ human cannabinoid CB1-myc-His6 receptor /Gi1 ternary complexes
PWR spectra of (A) WIN 55,212-2- or (B) CP 55,940-bound human cannabinoid CB1 receptor-containing proteolipid bilayers in the absence (1) or presence (2) of saturating concentrations of Gi1 protein (80 nM). (3) PWR spectra after addition of saturating concentration (200 nM) of GTPγS to the agonist/receptor/Gi1 ternary complexes. The figure shows only PWR spectra obtained with _s_-polarized light; similar results were obtained with _p_-polarization (not shown). The spectra are representative from three independent experiments.
Similar articles
- Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor.
Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, Selley DE. Paugh SW, et al. Mol Pharmacol. 2006 Jul;70(1):41-50. doi: 10.1124/mol.105.020552. Epub 2006 Mar 29. Mol Pharmacol. 2006. PMID: 16571654 - Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the cannabinoid agonist WIN 55,212-2.
Castelli MP, Paola Piras A, D'Agostino A, Pibiri F, Perra S, Gessa GL, Maccarrone M, Pistis M. Castelli MP, et al. Eur J Pharmacol. 2007 Nov 14;573(1-3):11-9. doi: 10.1016/j.ejphar.2007.06.047. Epub 2007 Jul 4. Eur J Pharmacol. 2007. PMID: 17644084 - Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2.
Fattore L, Viganò D, Fadda P, Rubino T, Fratta W, Parolaro D. Fattore L, et al. Eur J Neurosci. 2007 Apr;25(7):2191-200. doi: 10.1111/j.1460-9568.2007.05470.x. Eur J Neurosci. 2007. PMID: 17419755 - Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists.
Ashton JC, Wright JL, McPartland JM, Tyndall JD. Ashton JC, et al. Curr Med Chem. 2008;15(14):1428-43. doi: 10.2174/092986708784567716. Curr Med Chem. 2008. PMID: 18537620 Review. - Functional selectivity in cannabinoid signaling.
Varga EV, Georgieva T, Tumati S, Alves I, Salamon Z, Tollin G, Yamamura HI, Roeske WR. Varga EV, et al. Curr Mol Pharmacol. 2008 Nov;1(3):273-84. doi: 10.2174/1874467210801030273. Curr Mol Pharmacol. 2008. PMID: 20021440 Review.
Cited by
- Structural Insights into CB1 Receptor Biased Signaling.
Al-Zoubi R, Morales P, Reggio PH. Al-Zoubi R, et al. Int J Mol Sci. 2019 Apr 13;20(8):1837. doi: 10.3390/ijms20081837. Int J Mol Sci. 2019. PMID: 31013934 Free PMC article. Review. - The role of CXCR3/LRP1 cross-talk in the invasion of primary brain tumors.
Boyé K, Pujol N, D Alves I, Chen YP, Daubon T, Lee YZ, Dedieu S, Constantin M, Bello L, Rossi M, Bjerkvig R, Sue SC, Bikfalvi A, Billottet C. Boyé K, et al. Nat Commun. 2017 Nov 17;8(1):1571. doi: 10.1038/s41467-017-01686-y. Nat Commun. 2017. PMID: 29146996 Free PMC article. - Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?
Smith TH, Sim-Selley LJ, Selley DE. Smith TH, et al. Br J Pharmacol. 2010 Jun;160(3):454-66. doi: 10.1111/j.1476-5381.2010.00777.x. Br J Pharmacol. 2010. PMID: 20590557 Free PMC article. Review. - The potential for selective pharmacological therapies through biased receptor signaling.
Kenakin T. Kenakin T. BMC Pharmacol Toxicol. 2012 Aug 13;13:3. doi: 10.1186/2050-6511-13-3. BMC Pharmacol Toxicol. 2012. PMID: 22947056 Free PMC article. Review. - Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog.
Järbe TU, Li C, Liu Q, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2009 Apr;203(2):229-39. doi: 10.1007/s00213-008-1199-3. Epub 2008 Jun 3. Psychopharmacology (Berl). 2009. PMID: 18521574 Free PMC article.
References
- Altmann F, Staudacher E, Wilson IBH, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J. 1999;16:109–123. - PubMed
- Alves ID, Ciano KA, Boguslavski V, Varga E, Salamon Z, Yamamura HI, Hruby VJ, Tollin G. Selectivity, cooperativity, and reciprocity in the interactions between the delta-opioid receptor, its ligands, and G-proteins. J. Biol. Chem. 2004a;279:44673–44682. - PubMed
- Alves ID, Cowell SM, Salamon Z, Devanathan S, Tollin G, Hruby VJ. Different structural states of the proteolipid membrane are produced by ligand binding to the human delta-opioid receptor as shown by plasmon-waveguide resonance spectroscopy. Mol. Pharmacol. 2004b;65:1248–1257. - PubMed
- Alves ID, Salamon Z, Varga E, Yamamura HI, Tollin G, Hruby V. Direct observation of G-protein binding to the human delta-opioid receptor using plasmon-waveguide resonance spectroscopy. J. Biol. Chem. 2003;278:48890–48897. - PubMed
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006;105:1–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous