Foreign body reaction to biomaterials - PubMed (original) (raw)
Review
Foreign body reaction to biomaterials
James M Anderson et al. Semin Immunol. 2008 Apr.
Abstract
The foreign body reaction composed of macrophages and foreign body giant cells is the end-stage response of the inflammatory and wound healing responses following implantation of a medical device, prosthesis, or biomaterial. A brief, focused overview of events leading to the foreign body reaction is presented. The major focus of this review is on factors that modulate the interaction of macrophages and foreign body giant cells on synthetic surfaces where the chemical, physical, and morphological characteristics of the synthetic surface are considered to play a role in modulating cellular events. These events in the foreign body reaction include protein adsorption, monocyte/macrophage adhesion, macrophage fusion to form foreign body giant cells, consequences of the foreign body response on biomaterials, and cross-talk between macrophages/foreign body giant cells and inflammatory/wound healing cells. Biomaterial surface properties play an important role in modulating the foreign body reaction in the first two to four weeks following implantation of a medical device, even though the foreign body reaction at the tissue/material interface is present for the in vivo lifetime of the medical device. An understanding of the foreign body reaction is important as the foreign body reaction may impact the biocompatibility (safety) of the medical device, prosthesis, or implanted biomaterial and may significantly impact short- and long-term tissue responses with tissue-engineered constructs containing proteins, cells, and other biological components for use in tissue engineering and regenerative medicine. Our perspective has been on the inflammatory and wound healing response to implanted materials, devices, and tissue-engineered constructs. The incorporation of biological components of allogeneic or xenogeneic origin as well as stem cells into tissue-engineered or regenerative approaches opens up a myriad of other challenges. An in depth understanding of how the immune system interacts with these cells and how biomaterials or tissue-engineered constructs influence these interactions may prove pivotal to the safety, biocompatibility, and function of the device or system under consideration.
Figures
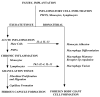
Figure 1
Sequence of events involved in inflammatory and wound healing responses leading to foreign body giant cell formation. This shows the potential importance of mast cells in the acute inflammatory phase and Th2 lymphocytes in the transient chronic inflammatory phase with the production of IL-4 and IL-13, which can induce monocyte/macrophage fusion to form foreign body giant cells.
Figure 2
In vivo transition from blood-borne monocyte to biomaterial adherent monocyte/macrophage to foreign body giant cell at the tissue/biomaterial interface. There is ongoing research to elucidate the biological mechanisms that are considered to play important roles in the transition to foreign body giant cell development.
Figure 3
Scanning electron microscopy images of an Elasthane 80A Polyurethane surface from an in vivo cage study showing the morphological progression of the foreign body reaction. The sequence of events at the Polyurethane surface includes (A) monocyte adhesion (0 days), (B) monocyte-to-macrophage development (3 days), (C) ongoing macrophage-macrophage fusion (7 days), and (D) foreign body giant cells (14 days).
Similar articles
- Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials.
Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Sheikh Z, et al. Materials (Basel). 2015 Aug 28;8(9):5671-5701. doi: 10.3390/ma8095269. Materials (Basel). 2015. PMID: 28793529 Free PMC article. Review. - Formation and biological activities of foreign body giant cells in response to biomaterials.
Cai F, Jiang B, He F. Cai F, et al. Acta Biomater. 2024 Oct 15;188:1-26. doi: 10.1016/j.actbio.2024.08.034. Epub 2024 Sep 7. Acta Biomater. 2024. PMID: 39245307 Review. - Molecular Characterization of Macrophage-Biomaterial Interactions.
Moore LB, Kyriakides TR. Moore LB, et al. Adv Exp Med Biol. 2015;865:109-22. doi: 10.1007/978-3-319-18603-0_7. Adv Exp Med Biol. 2015. PMID: 26306446 Free PMC article. Review. - Macrophage reaction against biomaterials in the mouse model - Phenotypes, functions and markers.
Klopfleisch R. Klopfleisch R. Acta Biomater. 2016 Oct 1;43:3-13. doi: 10.1016/j.actbio.2016.07.003. Epub 2016 Jul 6. Acta Biomater. 2016. PMID: 27395828 Review. - Foreign Body Reaction to Subcutaneous Implants.
Kastellorizios M, Tipnis N, Burgess DJ. Kastellorizios M, et al. Adv Exp Med Biol. 2015;865:93-108. doi: 10.1007/978-3-319-18603-0_6. Adv Exp Med Biol. 2015. PMID: 26306445 Review.
Cited by
- Leukocyte transmigration into tissue-engineered constructs is influenced by endothelial cells through Toll-like receptor signaling.
Bartaula-Brevik S, Pedersen TO, Blois AL, Papadakou P, Finne-Wistrand A, Xue Y, Bolstad AI, Mustafa K. Bartaula-Brevik S, et al. Stem Cell Res Ther. 2014 Dec 20;5(6):143. doi: 10.1186/scrt533. Stem Cell Res Ther. 2014. PMID: 25528303 Free PMC article. - Perspectives for Monocyte/Macrophage-Based Diagnostics of Chronic Inflammation.
Kzhyshkowska J, Gudima A, Moganti K, Gratchev A, Orekhov A. Kzhyshkowska J, et al. Transfus Med Hemother. 2016 Mar;43(2):66-77. doi: 10.1159/000444943. Epub 2016 Mar 15. Transfus Med Hemother. 2016. PMID: 27226789 Free PMC article. Review. - In vivo compatibility of graphene oxide with differing oxidation states.
Sydlik SA, Jhunjhunwala S, Webber MJ, Anderson DG, Langer R. Sydlik SA, et al. ACS Nano. 2015;9(4):3866-74. doi: 10.1021/acsnano.5b01290. Epub 2015 Apr 10. ACS Nano. 2015. PMID: 25849074 Free PMC article. - Hydrogen peroxide generation and biocompatibility of hydrogel-bound mussel adhesive moiety.
Meng H, Li Y, Faust M, Konst S, Lee BP. Meng H, et al. Acta Biomater. 2015 Apr;17:160-9. doi: 10.1016/j.actbio.2015.02.002. Epub 2015 Feb 10. Acta Biomater. 2015. PMID: 25676582 Free PMC article. - Spontaneous immunomodulation and regulation of angiogenesis and osteogenesis by Sr/Cu-borosilicate glass (BSG) bone cement to repair critical bone defects.
Li S, Zhang L, Liu C, Kim J, Su K, Chen T, Zhao L, Lu X, Zhang H, Cui Y, Cui X, Yuan F, Pan H. Li S, et al. Bioact Mater. 2022 Nov 9;23:101-117. doi: 10.1016/j.bioactmat.2022.10.021. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406252 Free PMC article.
References
- Anderson JM. Biological Responses to Materials. Annu Rev Mater Res. 2001;31:81–110.
- Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7(1):40–7. - PubMed
- Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669–87. - PubMed
- Luttikhuizen DT, Harmsen MC, Van Luyn MJ. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12(7):1955–70. - PubMed
- Horbett T. The role of adsorbed proteins in tissue response to biomaterials. In: Ratner B, et al., editors. Biomaterials Science: An Introduction to Biomaterials in Medicine. San Diego, CA: Elsevier Academic Press; 2004. pp. 237–46.
Publication types
MeSH terms
Substances
Grants and funding
- EB-000275/EB/NIBIB NIH HHS/United States
- R56 EB000282/EB/NIBIB NIH HHS/United States
- EB-000282/EB/NIBIB NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
- T32 GM07250/GM/NIGMS NIH HHS/United States
- R01 EB000282/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources