T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma - PubMed (original) (raw)
doi: 10.1016/j.immuni.2007.11.016. Epub 2007 Dec 27.
Bhanu P Pappu, Roza Nurieva, Askar Akimzhanov, Hong Soon Kang, Yeonseok Chung, Li Ma, Bhavin Shah, Athanasia D Panopoulos, Kimberly S Schluns, Stephanie S Watowich, Qiang Tian, Anton M Jetten, Chen Dong
Affiliations
- PMID: 18164222
- PMCID: PMC2587175
- DOI: 10.1016/j.immuni.2007.11.016
T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma
Xuexian O Yang et al. Immunity. 2008 Jan.
Abstract
T cell functional differentiation is mediated by lineage-specific transcription factors. T helper 17 (Th17) has been recently identified as a distinct Th lineage mediating tissue inflammation. Retinoic acid receptor-related orphan receptor gamma (ROR gamma) was shown to regulate Th17 differentiation; ROR gamma deficiency, however, did not completely abolish Th17 cytokine expression. Here, we report Th17 cells highly expressed another related nuclear receptor, ROR alpha, induced by transforming growth factor-beta and interleukin-6 (IL-6), which is dependent on signal transducer and activator of transcription 3. Overexpression of ROR alpha promoted Th17 differentiation, possibly through the conserved noncoding sequence 2 in Il17-Il17f locus. ROR alpha deficiency resulted in reduced IL-17 expression in vitro and in vivo. Furthermore, ROR alpha and ROR gamma coexpression synergistically led to greater Th17 differentiation. Double deficiencies in ROR alpha and ROR gamma globally impaired Th17 generation and completely protected mice against experimental autoimmune encephalomyelitis. Therefore, Th17 differentiation is directed by two lineage-specific nuclear receptors, ROR alpha and ROR gamma.
Figures
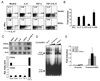
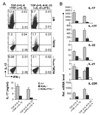
Figure 1. RORα is highly expressed in TH17 cells
(A) CD4+ T cells from OTII mice were differentiated with OVA323–339 peptide and splenic antigen presenting cells (APCs) under neutral (TH0), TH1, TH2 and TH17 conditions for 5 days, and RORα and RORγt mRNA expression levels were assessed by real-time RT-PCR after restimulation of T cells by anti-CD3 for 4 hours. Data shown are derived from 2 independent experiments with consistent results and normalized using expression levels of Actb. Expression levels in TH0 were referred as 1. (B) FACS-sorted naïve CD4+CD25−CD62LhiCD44lo T cells from C57BL/6 mice were activated with anti-CD3 and anti-CD28 in the presence of indicated cytokine stimuli for 1, 2 or 5 days, and RORα and RORγ mRNA expression levels were assessed by real-time RT-PCR. Expression levels in the condition without exogenous cytokine stimulus were referred as 1. (C) Naïve CD4+ T cells from STAT3 KO or wild-type (WT) mice were activated with plate-bound anti-CD3 and anti-CD28 under the indicated stimuli for 4–5 days. RORα mRNA expression levels were assessed by real-time RTPCR. The expression levels in STAT3 KO cells were referred as 1. The experiments were repeated at least 3 times with consistent results. *, student t test _p_≤0.05.
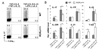
Figure 2. RORα overexpression promotes TH17 differentiation
(A) FACS-sorted naïve OT-II CD4+ T cells were activated with splenic APCs pulsed with OVA peptide in the presence of blocking antibodies for IL-4 and IFN-γ and the indicated stimuli and infected with an _IRES-GFP_-containing bicistronic retrovirus expressing RORα or a vector control virus. IL-17- and IFN-γ-expressing cells were measured by intracellular staining on a GFP+ gate. The experiments were repeated at least for three times with consistent results. (B) GFP+ cells were sorted from the above neutral culture conditions and mRNA expression was measured by real-time RT-PCR. Expression levels in cells transduced with the vector control virus were referred as 1. (C) Naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 in the presence of indicated stimuli for 2 days and histone H3 acetylation in CNS2 of IL-17-IL-17F locus was assessed by chromatin immuno-precipitation (ChIP) and PCR using two different pairs of primers (2a and 2b). Data were normalized to levels of histone H3 acetylation at the Actb promoter. (D) ROR binding to RORE at CNS2 was assessed by Electrophoretic Mobility Shift Assay. Nuclear proteins were prepared from TH17 cells differentiated from naïve OT-II T cells using splenic APC pulsed with OVA peptide in the presence of polarizing cytokines. A CNS2 RORE was used as probe. Competitors were a consensus element and a mutant of the CNS2 RORE. (E) RORα or RORγ overexpression enhances transcription from an IL-17 minimal promoter in the presence of CNS2. EL-4 cells were transfected with RORα, RORγ or both o0r vector alone with an IL-17 promoter (promoter) or _IL-17_-promoter-CNS2 (promoter+CNS2) luciferase reporter vector. Renilla luciferase was used to normalize transfection efficiency. Background luciferase activities in cells transfected with promoter reporter and empty vectors were referred as 1. Data shown represent two independent experiments with similar results.
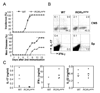
Figure 3. RORα deficiency reduces IL-17 production in vitro
(A) FACS-sorted naïve CD4+ T cells from WT or homozygous Staggerer (RORαsg/sg) were activated under the indicated conditions for 4 d. IL-17- or IFN-γ-expressing cells were measured by intracellular staining. The experiments were repeated at least for 3 times. (B) Total RNA was isolated from the above culture and mRNA expression for indicated genes was assessed by real-time RT-PCR. The lowest expression levels in each PCR were referred as 1. *, _p_≤0.05.
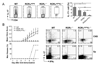
Figure 4. RORα deficiency reduces IL-17 production in vivo
(A) Rag1 KO mice were reconstituted with bone marrow from RORαsg/sg or WT mice. 6–8 weeks later, EAE was induced in the chimeric mice. Data shown are a combination of two independent experiments. WT, n=10; RORαsg/sg, n=9. Max disease, score ≥ 3. (B) Infiltrates in central nerve system or splenocytes from the EAE mice were isolated on day 14 after the 2nd immunization and restimulated with PMA/Ionomycin and MOG35–55 peptide, respectively. IL-17- or IFN-γ-expressing cells were measured by intracellular staining. Data shown are on gated CD4+ cells. CNS, central nerve system; Sp, spleen. (C) Splenocytes from the above mice were stimulated with MOG peptide and cytokine expression levels were measured by ELISA. Mean values were shown as horizontal bars. Data shown represent two independent experiments with consistent results.
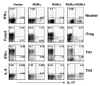
Figure 5. RORα and RORγt synergizes in promoting TH17 differentiation
Naïve OT-II CD4+ T cells were activated with OVA peptide-pulsed splenic APCs under the Neutral (anti-IL-4 and anti-IFN-γ); iTreg (inducible regulatory T cells, TGF-β, anti-IL-4 and anti-IFN-γ), TH1 or TH2 conditions and co-infected with two bicistronic retroviruses expressing RORα-GFP or GFP vector and RORγt-hCD2 or hCD2 vector. Cytokine- or Foxp3-expressing cells were assessed by intracellular staining. Data shown are gated on GFP+hCD2+ cells. The experiments were repeated at least twice with consistent results.
Figure 6. RORα and RORγ double deficiencies completely abrogate TH17 differentiation in vitro
Naïve CD4+CD25−CD62LhiCD44lo T cells from spleens of RORγ−/−, RORα-RORγ double deficient (RORαsg/sg/γ−/− or WT mice were activated with plated-bound anti-CD3 and anti-CD28 under the indicated conditions. (A) IL-17- and IFN-γ-secreting cells were assessed by intracellular staining. Protein levels of IL-17 were measured by ELISA after anti-CD3 restimulation. (B) Expression levels of indicated genes were measured by real-time RT-PCR. The lowest expression levels for each gene were referred as 1.
Figure 7. RORα and RORγ compound mutations completely inhibit TH17 differentiation in vivo
(A) Lamina propria cells were isolated from _Rag1_−/− mice reconstituted with RORαsg/sg, _RORγ_−/− RORαsg/sg/γ−/− or WT bone marrow cells and IL-17 expression was assessed by intracellular staining. Student t test, *, p<0.05; **, p<0.005. (B) EAE was induced in the indicated Rag1-deficient mice reconstituted with indicated bone marrow cells. WT, n=5; _RORγ_−/−, n=4; RORαsg/sg/γ−/−, n=5. Max disease, score ≥ 3. *, student t test RORαsg/sg/γ−/− vs WT, p<0.001; RORαsg/sg/γ−/− vs _RORγ_−/−, p<0.05. Infiltrates in central nerve system or splenocytes from the EAE mice were isolated on day 13 after the 2nd immunization and IL-17- or IFN-γ- expressing cells were measured by intracellular staining. Data shown are on gated CD4+ cells.
Comment in
- Regulation of T helper 17 differentiation by orphan nuclear receptors: it's not just ROR gamma t anymore.
Sundrud MS, Rao A. Sundrud MS, et al. Immunity. 2008 Jan;28(1):5-7. doi: 10.1016/j.immuni.2007.12.006. Immunity. 2008. PMID: 18199410
References
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. - PubMed
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. - PubMed
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
- Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases