Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly - PubMed (original) (raw)
Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly
Viviane Poupon et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Clathrin-coated vesicles (CCVs) are major carriers for endocytic cargo and mediate important intracellular trafficking events at the trans-Golgi network (TGN) and endosomes. Whereas clathrin heavy chain provides the structural backbone of the clathrin coat, the role of clathrin light chains (CLCs) is poorly understood. We now demonstrate that CLCs are not required for clathrin-mediated endocytosis but are critical for clathrin-mediated trafficking between the TGN and the endosomal system. Specifically, CLC knockdown (KD) causes the cation-independent mannose-6 phosphate receptor (CI-MPR) to cluster near the TGN leading to a delay in processing of the lysosomal hydrolase cathepsin D. A recently identified binding partner for CLCs is huntingtin-interacting protein 1-related (HIP1R), which is required for productive interactions of CCVs with the actin cytoskeleton. CLC KD causes mislocalization of HIP1R and overassembly of actin, which accumulates in patches around the clustered CI-MPR. A dominant-negative CLC construct that disrupts HIP1R/CLC interactions causes similar alterations in CI-MPR trafficking and actin assembly. Thus, in mammalian cells CLCs function in intracellular membrane trafficking by acting as recruitment proteins for HIP1R, enabling HIP1R to regulate actin assembly on clathrin-coated structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
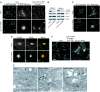
CLC KD disrupts CI-MPR trafficking. (A) HeLa or HeLaM cells (expressing CD8–CI-MPR), transfected without (Mock) or with CLCa and CLCb siRNAs for 96 h, were processed for immunofluorescence with antibodies for CI-MPR or the CD8 tag. Higher-magnification images in Center correspond to areas marked by white squares in the lower-magnification images in Left. [Scale bar: 10 μm (Left and Right) and 2 μm (Center).] (B) HeLa and COS-7 cell lysates were prepared 96 h after transfection without (Mock) or with CLCa and CLCb siRNAs, and equal protein aliquots were processed for Western blot with antibodies for the indicated proteins. (C) Immunofluorescence analysis of AP-1 in mock-transfected COS-7 cells and cells transfected with CLCa and CLCb siRNA. Higher-magnification images in Right correspond to areas marked by white squares in the lower-magnification images in Left. [Scale bar: 10 μm (Left) and 2 μm (Right).] (D) Mock-transfected or CLCa and CLCb siRNA-transfected COS-7 cells were processed for immunofluorescence with antibodies for CI-MPR (red) and TGN46 (green). (Scale bar: 2 μm.) (E) HeLa cells, mock-transfected or transfected with CLCa and CLCb siRNA, were processed for conventional transmission EM. (Scale bar: 100 nm.) The arrows indicate CCVs. (F) HeLaM cells expressing CD8–CI-MPR chimera were transfected without siRNA (Mock) or with CLCa and CLCb siRNAs for 96 h. Live cells were then placed at 4°C and exposed to CD8 mAb for 30 min followed by a switch to 37°C for 3 h to allow receptor/antibody internalization. Cells were then fixed and processed for immunofluorescence with secondary antibody to detect the CD8 antibody. (Scale bar: 10 μm.)
Fig. 2.
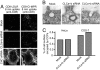
Endocytic function is normal after CLC KD. (A) Mock- and siRNA-treated HeLaM cells, stably expressing CD8–LDLR or CD8–CI-MPR chimeras, were allowed to endocytose the anti-CD8 antibody for 8 min and were then fixed and processed for immunofluorescence with secondary antibody to detect the CD8 antibody. (Scale bar: 10 μm.) (B) Mock- and CLCa/CLCb siRNA-treated HeLa cells were processed for conventional transmission EM. (Scale bar: 100 nm.) (C) Multiple randomly selected EM images from mock- or CLCa and CLCb siRNA-transfected HeLa and COS-7 cells were overlaid with a 500-nm grid. The number of PM and CCP intersections, respectively, were as follows: HeLa (mock), 7,211 and 40; HeLa (CLCs siRNA), 6,819 and 38; COS-7 (mock), 8,056 and 55; COS-7 (CLCs siRNA), 7,211 and 58.
Fig. 3.
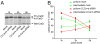
CLC KD delays processing of cathepsin D. (A) Mock- or CLCa and CLCb siRNA-transfected COS-7 cells were pulsed for 2 h at 20°C with 35S-labeled methionine/cysteine followed by a chase with unlabeled methionine. At the indicated time points, cathepsin D was immunoprecipitated from cell lysates and analyzed by SDS/PAGE and autoradiography. (B) The percentage of the pro- and intermediate forms of cathepsin D compared with total cathepsin D signal is plotted for mock- and CLCa and CLCb siRNA-transfected cells at each time point (mean ± SEM of n = 8 experiments).
Fig. 4.
Disruption of CLC function alters the actin cytoskeleton. (A) Mock- or CLCa and CLCb siRNA-transfected HeLa cells were processed for immunofluorescence with antibodies for CLC, and actin distribution was assessed by using phalloidin-TRITC. Higher-magnification images correspond to the areas marked by the white squares in the lower-magnification images. (Scale bar: 10 μm and 2 μm for the low- and high-magnification images, respectively.) (B) HeLa cells were transfected with GFP-CLCb or GFP-CLCb EED to QQN mutant (CLCb QQN). At 48 h after transfection, cells were fixed and processed to detect actin by using phalloidin-TRITC (red). Higher-magnification images correspond to areas marked by white squares in the lower-magnification images. (Scale bar: 10 μm and 2 μm for the low- and high-magnification images, respectively.) (C) CLCa and CLCb siRNA-transfected HeLa cells were processed for immunofluorescence with antibodies for CLC (green) and CI-MPR (blue), and actin distribution was assessed by using phalloidin-TRITC (red). Higher-magnification images correspond to the area marked by white squares in the lower-magnification images. (Scale bar: 10 μm and 4 μm for the low- and high-magnification images, respectively.)
Fig. 5.
CLC KD disrupts the levels and distribution of HIP1R. (A) Cell lysates from mock- or CLCa and CLCb-transfected cells were processed for Western blot with antibodies for the indicated proteins. Quantification reveals a 25 ± 3% (mean ± SEM, n = 12) decrease in HIP1R levels in CLC KD versus mock-transfected cells. (B) Immunofluorescence analysis of the localization of HIP1R in mock-transfected COS-7 cells and cells transfected with CLCa and CLCb siRNA. (Scale bar: 10 μm.) (C) Mock- or CLCa and CLCb siRNA-transfected HeLa cells were subsequently transfected with plasmid encoding HIP1R and processed for immunofluorescence with antibodies for HIP1R (green) and CLCs (blue), and actin distribution was assessed by using phalloidin-TRITC (red).
Similar articles
- Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network.
Carreno S, Engqvist-Goldstein AE, Zhang CX, McDonald KL, Drubin DG. Carreno S, et al. J Cell Biol. 2004 Jun 21;165(6):781-8. doi: 10.1083/jcb.200403120. J Cell Biol. 2004. PMID: 15210728 Free PMC article. - Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging.
Ito E, Fujimoto M, Ebine K, Uemura T, Ueda T, Nakano A. Ito E, et al. Plant J. 2012 Jan;69(2):204-16. doi: 10.1111/j.1365-313X.2011.04782.x. Epub 2011 Oct 14. Plant J. 2012. PMID: 21910772 - Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model.
Robinson DG, Neuhaus JM. Robinson DG, et al. J Exp Bot. 2016 Aug;67(15):4435-49. doi: 10.1093/jxb/erw222. Epub 2016 Jun 4. J Exp Bot. 2016. PMID: 27262127 Review. - Clathrin: its role in receptor-mediated vesicular transport and specialized functions in neurons.
Pley U, Parham P. Pley U, et al. Crit Rev Biochem Mol Biol. 1993;28(5):431-64. doi: 10.3109/10409239309078441. Crit Rev Biochem Mol Biol. 1993. PMID: 8269710 Review.
Cited by
- Myosin 1 controls membrane shape by coupling F-Actin to membrane.
Coudrier E, Almeida CG. Coudrier E, et al. Bioarchitecture. 2011 Sep 1;1(5):230-235. doi: 10.4161/bioa.18406. Bioarchitecture. 2011. PMID: 22754614 Free PMC article. - Clathrin light chains are required for the gyrating-clathrin recycling pathway and thereby promote cell migration.
Majeed SR, Vasudevan L, Chen CY, Luo Y, Torres JA, Evans TM, Sharkey A, Foraker AB, Wong NM, Esk C, Freeman TA, Moffett A, Keen JH, Brodsky FM. Majeed SR, et al. Nat Commun. 2014 May 23;5:3891. doi: 10.1038/ncomms4891. Nat Commun. 2014. PMID: 24852344 Free PMC article. - PCSK9 signaling pathways and their potential importance in clinical practice.
Wiciński M, Żak J, Malinowski B, Popek G, Grześk G. Wiciński M, et al. EPMA J. 2017 Aug 14;8(4):391-402. doi: 10.1007/s13167-017-0106-6. eCollection 2017 Dec. EPMA J. 2017. PMID: 29209441 Free PMC article. Review. - Imaging endocytic clathrin structures in living cells.
Kirchhausen T. Kirchhausen T. Trends Cell Biol. 2009 Nov;19(11):596-605. doi: 10.1016/j.tcb.2009.09.002. Trends Cell Biol. 2009. PMID: 19836955 Free PMC article. Review. - Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route.
Poirier S, Mayer G, Poupon V, McPherson PS, Desjardins R, Ly K, Asselin MC, Day R, Duclos FJ, Witmer M, Parker R, Prat A, Seidah NG. Poirier S, et al. J Biol Chem. 2009 Oct 16;284(42):28856-64. doi: 10.1074/jbc.M109.037085. Epub 2009 Jul 27. J Biol Chem. 2009. PMID: 19635789 Free PMC article.
References
- Kirchhausen T, Harrison SC. Cell. 1981;23:755–761. - PubMed
- Ungewickell E, Branton D. Nature. 1981;289:420–422. - PubMed
- Girard M, Allaire PD, McPherson PS, Blondeau F. Mol Cell Proteomics. 2005;4:1145–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous