Genome-wide expression analysis of a spinal muscular atrophy model: towards discovery of new drug targets - PubMed (original) (raw)
Genome-wide expression analysis of a spinal muscular atrophy model: towards discovery of new drug targets
Sheena Lee et al. PLoS One. 2008.
Erratum in
- PLoS ONE. 2008;3(4). doi: 10.1371/annotation/f45a5dc0-e317-4886-8c5b-1569c79a5f08. Cauchi, Ruben J [added]
Abstract
Spinal Muscular Atrophy is a recessive genetic disease and affects lower motor neurones and muscle tissue. A single gene is disrupted in SMA: SMN1 activity is abolished but a second copy of the gene (SMN2) provides limited activity. While the SMN protein has been shown to function in the assembly of RNA-protein complexes, it is unclear how the overall reduction in SMN activity specifically results in the neuromuscular phenotypes. Similar to humans, reduced smn activity in the fly causes earliest phenotypes in neuromuscular tissues. To uncover the effects of reduced SMN activity, we have studied gene expression in control and diseased fly tissues using whole genome micro-arrays. A number of gene expression changes are recovered and independently validated. Identified genes show trends in their predicted function: several are consistent with the function of SMN, in addition some uncover novel pathways. This and subsequent genetic analysis in the fly indicates some of the identified genes could be taken for further studies as potential drug targets for SMA and other neuromuscular disorders.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
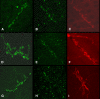
Figure 1. Confocal Scanning Fluorescent images of Drosophila neuromuscular junctions.
A to C, early second instar larvae; D to F, late second instar larvae and G to I, third instar larvae (mid stage). A, D and G, normal (+/+) larvae, B, C, E, F, H and I, smn mutant larvae (smn/smn). A, B, D, E, G and H stained for Glutamate receptor subunit; C, F and I stained for a pre-synaptic marker (syntaxin). Comparing the accumulation of receptor clusters in the normal tissue across time to the mutant tissues, it is clear that the mutant displays a reduced number of glutamate receptor clusters across time.
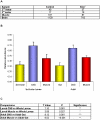
Figure 2. Smn gene expression analysis using two different methods.
A) Results of micro-array hybridisations. “Sample” column lists the different RNA samples used for cRNA preparation. The control and smn mutant relative concentrations are shown. Expression is low in the RNA samples derived from whole animals but is significantly higher in neuromuscular tissues, especially in brain. The expression is reduced by 1.9 and 1.5 fold in the mutant animals in muscle and brain respectively. Note that these tissues are mutant for the somatic gene (a point mutation so transcripts will be produced) while still containing detectable maternal RNA (data not shown). B) Real Time-PCR analysis of smn expression across different tissues. The Bar figure shows the relative abundance of smn transcript in tissues versus either whole animals (thus containing the isolated tissues; all third instar larval stage derived) or versus isolated gut tissue (all adult derived), all from smn +/+. In both Central Nervous System (CNS) and muscle the expression of smn is higher. C) The significance of this finding increases in adults
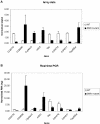
Figure 3. Bar graphs showing the gene expression changes across a set of eight genes comparing Affymetrix micro-array hybridisation (top) and Real-Time Polymerase Chain Reaction (bottom) results in whole 3rd instar larvae and muscle.
Sample size was three for larvae and four for muscle. The eight genes were CG6776, CG8836, Cyp4ac3, mthl3, Gip, CG4778, CG4511 and Tsp42Ed. The changes measured this way showed that mRNA synthesis in whole 3rd instar SMN mutant larvae compared to WT larvae for CG6776 was decreased 3.57 fold (P<0.01), CG8836 was increased 14.6 fold (P<0.05), Cyp4ac3 was increased 2.7 fold (P<0.05), mthl3 was increased 8.9 fold (P<0.05). mRNA synthesis in SMN mutant larval muscle for Gip was decreased 2.7 fold (P<0.01), CG4778 was decreased 2.7 fold (
<p0.01), cg4511="" was="" decreased="" 1.6="" fold="" (p<0.05),="" tsp42ed="" increased="" 21.6="" (p<0.05)="" .="" the="" rt-pcr="" analysis="" validates="" micro-array="" data:="" overall="" directions="" of="" change="" are="" all="" identical="" and="" changes="" within="" same="" range<="" div=""> </p0.01),>
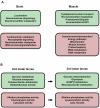
Figure 4. A) A selection of Gene Ontology terms associated with up (red)- or down (green) -regulated genes in muscle and brain tissues, comparing normal with smn mutant.
B) A selection of Gene Ontology terms associated with up- or down-regulated genes in 2nd and 3rd instar larvae, comparing normal with smn mutant, same colour-coding. For both A) and B) full lists of these terms are shown in Supplemental Figure S3.
Similar articles
- Robust quantification of the SMN gene copy number by real-time TaqMan PCR.
Gómez-Curet I, Robinson KG, Funanage VL, Crawford TO, Scavina M, Wang W. Gómez-Curet I, et al. Neurogenetics. 2007 Nov;8(4):271-8. doi: 10.1007/s10048-007-0093-1. Epub 2007 Jul 24. Neurogenetics. 2007. PMID: 17647030 - Molecular and functional analysis of intragenic SMN1 mutations in patients with spinal muscular atrophy.
Sun Y, Grimmler M, Schwarzer V, Schoenen F, Fischer U, Wirth B. Sun Y, et al. Hum Mutat. 2005 Jan;25(1):64-71. doi: 10.1002/humu.20111. Hum Mutat. 2005. PMID: 15580564 - Therapeutics development for spinal muscular atrophy.
Sumner CJ. Sumner CJ. NeuroRx. 2006 Apr;3(2):235-45. doi: 10.1016/j.nurx.2006.01.010. NeuroRx. 2006. PMID: 16554261 Free PMC article. Review. - Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy.
Thurmond J, Butchbach ME, Palomo M, Pease B, Rao M, Bedell L, Keyvan M, Pai G, Mishra R, Haraldsson M, Andresson T, Bragason G, Thosteinsdottir M, Bjornsson JM, Coovert DD, Burghes AH, Gurney ME, Singh J. Thurmond J, et al. J Med Chem. 2008 Feb 14;51(3):449-69. doi: 10.1021/jm061475p. Epub 2008 Jan 19. J Med Chem. 2008. PMID: 18205293 - Spinal muscular atrophy: from gene to therapy.
Wirth B, Brichta L, Hahnen E. Wirth B, et al. Semin Pediatr Neurol. 2006 Jun;13(2):121-31. doi: 10.1016/j.spen.2006.06.008. Semin Pediatr Neurol. 2006. PMID: 17027862 Review.
Cited by
- Genetic screen identifies a requirement for SMN in mRNA localisation within the Drosophila oocyte.
Aquilina B, Cauchi RJ. Aquilina B, et al. BMC Res Notes. 2018 Jun 13;11(1):378. doi: 10.1186/s13104-018-3496-1. BMC Res Notes. 2018. PMID: 29895323 Free PMC article. - Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick?
Burghes AH, Beattie CE. Burghes AH, et al. Nat Rev Neurosci. 2009 Aug;10(8):597-609. doi: 10.1038/nrn2670. Epub 2009 Jul 8. Nat Rev Neurosci. 2009. PMID: 19584893 Free PMC article. Review. - Modeling spinal muscular atrophy in Drosophila.
Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, Artavanis-Tsakonas S. Chang HC, et al. PLoS One. 2008 Sep 15;3(9):e3209. doi: 10.1371/journal.pone.0003209. PLoS One. 2008. PMID: 18791638 Free PMC article. - Motor defects in a Drosophila model for spinal muscular atrophy result from SMN depletion during early neurogenesis.
Grice SJ, Liu JL. Grice SJ, et al. PLoS Genet. 2022 Jul 25;18(7):e1010325. doi: 10.1371/journal.pgen.1010325. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35877682 Free PMC article. - Plexin-Semaphorin Signaling Modifies Neuromuscular Defects in a Drosophila Model of Peripheral Neuropathy.
Grice SJ, Sleigh JN, Zameel Cader M. Grice SJ, et al. Front Mol Neurosci. 2018 Feb 22;11:55. doi: 10.3389/fnmol.2018.00055. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29520219 Free PMC article.
References
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. - PubMed
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nature Genetics. 1997;16:265–269. - PubMed
- Schrank B, Gotz R, Gunnersen JM, Ure JM, Toyka KV, et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9920–9925. - PMC - PubMed
- Bergin A, Kim G, Price DL, Sisodia SS, Lee MK, et al. Identification and characterization of a mouse homologue of the spinal muscular atrophy-determining gene, survival motor neuron. Gene. 1997;204:47–53. - PubMed
- Yong J, Wan L, Dreyfuss G. Why do cells need an assembly machine for RNA-protein complexes? Trends in cell biology. 2004;14:226–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical