Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae - PubMed (original) (raw)
Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae
James H Soper et al. Mol Biol Cell. 2008 Mar.
Erratum in
- Mol Biol Cell. 2008 May;19(5):2348
Abstract
Aggregated alpha-synuclein (alpha-syn) fibrils form Lewy bodies (LBs), the signature lesions of Parkinson's disease (PD) and related synucleinopathies, but the pathogenesis and neurodegenerative effects of LBs remain enigmatic. Recent studies have shown that when overexpressed in Saccharomyces cerevisiae, alpha-syn localizes to plasma membranes and forms cytoplasmic accumulations similar to human alpha-syn inclusions. However, the exact nature, composition, temporal evolution, and underlying mechanisms of yeast alpha-syn accumulations and their relevance to human synucleinopathies are unknown. Here we provide ultrastructural evidence that alpha-syn accumulations are not comprised of LB-like fibrils, but are associated with clusters of vesicles. Live-cell imaging showed alpha-syn initially localized to the plasma membrane and subsequently formed accumulations in association with vesicles. Imaging of truncated and mutant forms of alpha-syn revealed the molecular determinants and vesicular trafficking pathways underlying this pathological process. Because vesicular clustering is also found in LB-containing neurons of PD brains, alpha-syn-mediated vesicular accumulation in yeast represents a model system to study specific aspects of neurodegeneration in PD and related synucleinopathies.
Figures
Figure 1.
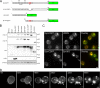
α-Syn-EGFP expression and accumulation in S. cerevisiae. (A) Schematic of α-syn-EGFP constructs used in this study. Locations of KTKEGV motif repeats, the hydrophobic residues 71-82 within the NAC domain, A30P and A53T point mutations, and truncated/deleted regions are indicated. (B) Immunoblot analysis of yeast extracts from cells expressing PYES2 constructs using a C-terminal anti-α-syn mAb (LB509), an N-terminal anti-α-syn mAb (Syn303), an anti-GFP antibody, an anti-β-syn mAb (Syn 207), and an α-tubulin antibody. (C) α-Syn immunofluorescence on yeast expressing α-syn-EGFP using LB509 and Syn303. Both mAb immunostaining colocalize completely with the GFP signal, indicating that the entire fusion protein is present. (D) Time-lapse imaging of α-syn-EGFP (PYES2 Syn-EGFP) accumulation formation in yeast. Images were taken every 80 min starting when syn-EGFP protein expression was visible. Membrane-associated accumulations begin to form at 80 min (arrow) and grow larger in size. Some accumulations appear to coalesce to form larger accumulations (arrowheads). For corresponding video, see Supplementary Information, Video1 online. Scale bars, (C) 2 μm; (D) 1 μm.
Figure 2.
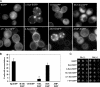
Structural requirements for α-syn-EGFP accumulation and toxicity in yeast. (A) Effect of deletions and mutations on α-syn-EGFP localization in yeast. EGFP alone has a diffuse localization, whereas WT α-syn-EGFP binds the plasma membrane and forms membrane associated accumulations. Deletion of the N-terminus (58-140-EGFP) eliminates membrane binding and α-syn accumulation, whereas expression of only the N-terminus (1-57-EGFP) preserves membrane binding but eliminates α-syn accumulation. The deletion of residues 71-82 (Δ71-82-EGFP), a region important for α-syn fibril formation, showed a dramatic reduction in the number of cells with α-syn accumulation although membrane localization is not impaired. Similarly, β-syn-EGFP also binds membrane but does not accumulate in the cytoplasm. A30P-EGFP shows membrane localization and cytoplasmic distribution but does not accumulate. (B) Quantification of the percentage of cells containing accumulations in yeast expressing different deletions and mutations of α-syn-EGFP. Error bars, SD. (C) Effect of various deletions on growth of yeast. Only cells expressing full-length α-syn have a growth defect. Scale bar, (A) 1 μm.
Figure 3.
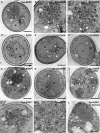
α-Syn-EGFP accumulations are composed of vesicles. Electron microscopy on yeast expressing α-syn-EGFP shows dense accumulations of vesicles (A–C), which do not appear in cells expressing EGFP alone (D). Cells expressing Δ71-82-EGFP (E), 1-57-EGFP (F), and 58-140-EGFP (H) do not have vesicular accumulations. Cells expressing A53T-EGFP have vesicular accumulations that are similar to wild type (G). Cells expressing α-synuclein without DMSO treatment formed similar accumulations (I). Immuno-EM using SNL-1, a polyclonal anti-α-syn antibody with 10-nm colloidal gold, shows that α-syn localizes to the vesicular accumulations (J–L), as well as the plasma membrane (J and L). Omission of osmication resulted in stronger antibody staining (L). Cells expressing EGFP alone had no staining with the anti-α-syn antibody (D), whereas cells expressing 58-140-EGFP displayed cytoplasmic staining (I). N, nucleus. Scale bars, (A, D–I, and L) 500 nm; (B and J) 100 nm; (C and K) 50 nm.
Figure 4.
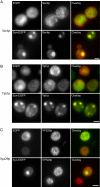
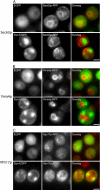
α-Syn-EGFP vesicular accumulations are colocalized with Rab GTPases Sec4p and Ypt1p. (A) α-Syn-EGFP accumulations are colocalized with Sec4p. 3HA-tagged-Sec4p was overexpressed (pRS413 3HA-Sec4p) in cells expressing either EGFP or α-syn-EGFP. In cells expressing EGFP or in α-syn-EGFP cells without accumulations, Sec4p was localized to small punctate structures throughout the cytoplasm (secretory vesicles). In cells with α-syn-EGFP accumulations, Sec4p staining was strongly colocalized with these accumulations. (B) α-Syn-EGFP accumulations are colocalized with Ypt1p. 3HA-tagged-Ypt1p was overexpressed (pRS413 3HA-Ypt1p) in cells expressing either EGFP or syn-EGFP. Cells were immunostained with anti-HA antibody. In cells expressing EGFP or α-syn-EGFP cells without accumulations, Ypt1p is localized to small punctate structures (most likely ER-Golgi transport vesicles) throughout the cytoplasm, whereas in cells with α-syn-EGFP accumulations, Ypt1p colocalizes with these accumulations. (C) 3HA-tagged-Vps29p did not colocalize with α-syn-EGFP accumulations, and localization of Vps29p was similar in cells expressing EGFP and α-syn-EGFP. Scale bar, 2 μm.
Figure 5.
α-Syn-EGFP accumulations do not disrupt organization of the ER, vacuole, or Golgi. (A) Presence of α-syn-EGFP accumulations does not disrupt localization of the ER marker Sec63p. Sec63p was C-terminally tagged with monomeric RFP. Expression of EGFP or α-syn-EGFP shows similar localization of the ER marker. (B) Presence of α-syn-EGFP accumulations does not disrupt localization of the vacuole membrane marker Vma4p, which was c-terminally tagged with monomeric RFP. (C) Presence of α-syn-EGFP accumulations does not disrupt localization of the endosomal marker, Vps17p, which was c-terminally tagged with dimeric RFP. Scale bar, 2 μm.
Figure 6.
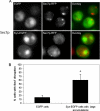
Presence of α-syn-EGFP accumulations disrupts localization of the Golgi marker Sec7p. (A) Presence of α-syn-EGFP accumulations disrupt localization of the late Golgi marker Sec7p, which was C-terminally tagged with monomeric RFP. Scale bar, 2 μm (B) Quantification of Sec7p disruption in cells expressing EGFP and cells expressing α-syn-EGFP with large accumulations. Scale bar, 2 μm.
Figure 7.
Presence of vesicular accumulations in human PD LBs. EM micrographs of LBs from the substantia nigra of a PD patient. LBs were composed of a dense fibrillar core with associated small vesicular accumulations (arrow) around the perimeter (A–E). Examination of sites more distal to the LBs revealed less dense distribution of small vesicles than that seen around the LB perimeter (F). Scale bars, (A, C, and E) 2 μm. (B, D, and F) 500 nm.
Similar articles
- The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis.
Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. Gitler AD, et al. Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):145-50. doi: 10.1073/pnas.0710685105. Epub 2007 Dec 27. Proc Natl Acad Sci U S A. 2008. PMID: 18162536 Free PMC article. - Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells.
Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Luk KC, et al. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20051-6. doi: 10.1073/pnas.0908005106. Epub 2009 Nov 5. Proc Natl Acad Sci U S A. 2009. PMID: 19892735 Free PMC article. - Alpha-Synuclein and the Endolysosomal System in Parkinson's Disease: Guilty by Association.
Teixeira M, Sheta R, Idi W, Oueslati A. Teixeira M, et al. Biomolecules. 2021 Sep 9;11(9):1333. doi: 10.3390/biom11091333. Biomolecules. 2021. PMID: 34572546 Free PMC article. Review. - Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.
Sahay S, Ghosh D, Singh PK, Maji SK. Sahay S, et al. Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review. - Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models.
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Cooper AA, et al. Science. 2006 Jul 21;313(5785):324-8. doi: 10.1126/science.1129462. Epub 2006 Jun 22. Science. 2006. PMID: 16794039 Free PMC article.
Cited by
- The Role of Alpha-Synuclein and Other Parkinson's Genes in Neurodevelopmental and Neurodegenerative Disorders.
Morato Torres CA, Wassouf Z, Zafar F, Sastre D, Outeiro TF, Schüle B. Morato Torres CA, et al. Int J Mol Sci. 2020 Aug 10;21(16):5724. doi: 10.3390/ijms21165724. Int J Mol Sci. 2020. PMID: 32785033 Free PMC article. Review. - Endogenous alpha-synuclein monomers, oligomers and resulting pathology: let's talk about the lipids in the room.
Killinger BA, Melki R, Brundin P, Kordower JH. Killinger BA, et al. NPJ Parkinsons Dis. 2019 Nov 12;5:23. doi: 10.1038/s41531-019-0095-3. eCollection 2019. NPJ Parkinsons Dis. 2019. PMID: 31728405 Free PMC article. Review. - Plasticity of Membrane Binding by the Central Region of α-Synuclein.
Navarro-Paya C, Sanz-Hernandez M, De Simone A. Navarro-Paya C, et al. Front Mol Biosci. 2022 Jun 15;9:857217. doi: 10.3389/fmolb.2022.857217. eCollection 2022. Front Mol Biosci. 2022. PMID: 35782868 Free PMC article. - All Roads Lead to Rome: Different Molecular Players Converge to Common Toxic Pathways in Neurodegeneration.
Argueti-Ostrovsky S, Alfahel L, Kahn J, Israelson A. Argueti-Ostrovsky S, et al. Cells. 2021 Sep 16;10(9):2438. doi: 10.3390/cells10092438. Cells. 2021. PMID: 34572087 Free PMC article. Review. - Alpha-synuclein Toxicity in the Early Secretory Pathway: How It Drives Neurodegeneration in Parkinsons Disease.
Wang T, Hay JC. Wang T, et al. Front Neurosci. 2015 Nov 12;9:433. doi: 10.3389/fnins.2015.00433. eCollection 2015. Front Neurosci. 2015. PMID: 26617485 Free PMC article. Review.
References
- Abeliovich A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O. M., Sudhof T. C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
- Chartier-Harlin M. C., et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. - PubMed
- Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 2002;277:6344–6352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous