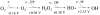Cellular defenses against superoxide and hydrogen peroxide - PubMed (original) (raw)
Review
Cellular defenses against superoxide and hydrogen peroxide
James A Imlay. Annu Rev Biochem. 2008.
Abstract
Life evolved in an anaerobic world; therefore, fundamental enzymatic mechanisms and biochemical pathways were refined and integrated into metabolism in the absence of any selective pressure to avoid reactivity with oxygen. After photosystem II appeared, environmental oxygen levels rose very slowly. During this time, microorganisms acquired oxygen tolerance by jettisoning enzymes that use glycyl radicals and exposed low-potential iron-sulfur clusters, which can be directly poisoned by oxygen. They also developed mechanisms to defend themselves against superoxide (O(2)()) and hydrogen peroxide, partially reduced oxygen species that are generated as inadvertent by-products of aerobic metabolism. Contemporary organisms have inherited both the vulnerabilities and the defenses of these ancestral microbes. Current research seeks to identify these, and bacteria comprise an exceptionally accessible experimental system that has provided many of the answers. This manuscript reviews recent developments and identifies remaining puzzles.
Figures
Fig. 1
The redox states of oxygen with standard reduction potentials. The standard concentration of oxygen was regarded as 1 M.
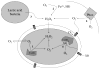
Fig. 2
Sources of oxidative stress for bacteria include (1) intracellular enzyme autoxidation, (2) environmental redox reactions, (3) H2O2 released by competing microbes, (4) phagosomal NADPH oxidase, and (5) redox-cycling antibiotics.
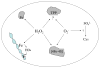
Fig. 3
Known mechanisms by which H2O2 and O2− injure cells include Fenton-mediated damage to proteins and DNA, the oxidation of solvent-exposed [4Fe-4S] clusters, apparent inhibition of transketolase, and disruption of the sulfur assimilatory pathway. The details of inactivation of transketolase and of sulfur metabolism remain unclear.
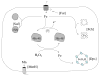
Fig. 4
Metal metabolism is altered during H2O2 stress. The Suf system restores cluster assembly, Dps sequesters unincorporated iron, Fur represses iron import, and ferrochetalase (Fch) ensures continued heme activation. The basis of protection by induced manganese import is uncertain.
Similar articles
- Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli.
Park S, You X, Imlay JA. Park S, et al. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9317-22. doi: 10.1073/pnas.0502051102. Epub 2005 Jun 20. Proc Natl Acad Sci U S A. 2005. PMID: 15967999 Free PMC article. - How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis.
Imlay JA. Imlay JA. Adv Microb Physiol. 2002;46:111-53. doi: 10.1016/s0065-2911(02)46003-1. Adv Microb Physiol. 2002. PMID: 12073652 Review. - OxyR and SoxRS regulation of fur.
Zheng M, Doan B, Schneider TD, Storz G. Zheng M, et al. J Bacteriol. 1999 Aug;181(15):4639-43. doi: 10.1128/JB.181.15.4639-4643.1999. J Bacteriol. 1999. PMID: 10419964 Free PMC article. - Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments.
Iuchi S, Weiner L. Iuchi S, et al. J Biochem. 1996 Dec;120(6):1055-63. doi: 10.1093/oxfordjournals.jbchem.a021519. J Biochem. 1996. PMID: 9010748 Review. - Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells.
Hassett DJ, Cohen MS. Hassett DJ, et al. FASEB J. 1989 Dec;3(14):2574-82. doi: 10.1096/fasebj.3.14.2556311. FASEB J. 1989. PMID: 2556311 Review.
Cited by
- Intracellular signaling by diffusion: can waves of hydrogen peroxide transmit intracellular information in plant cells?
Vestergaard CL, Flyvbjerg H, Møller IM. Vestergaard CL, et al. Front Plant Sci. 2012 Dec 31;3:295. doi: 10.3389/fpls.2012.00295. eCollection 2012. Front Plant Sci. 2012. PMID: 23293647 Free PMC article. - DNA Phosphorothioate Modification Plays a Role in Peroxides Resistance in Streptomyces lividans.
Dai D, Du A, Xiong K, Pu T, Zhou X, Deng Z, Liang J, He X, Wang Z. Dai D, et al. Front Microbiol. 2016 Aug 31;7:1380. doi: 10.3389/fmicb.2016.01380. eCollection 2016. Front Microbiol. 2016. PMID: 27630631 Free PMC article. - Effects of intracellular Mn on the radiation resistance of the halophilic archaeon Halobacterium salinarum.
Webb KM, Yu J, Robinson CK, Noboru T, Lee YC, DiRuggiero J. Webb KM, et al. Extremophiles. 2013 May;17(3):485-97. doi: 10.1007/s00792-013-0533-9. Epub 2013 Mar 27. Extremophiles. 2013. PMID: 23532412 - Antioxidant therapeutics: Pandora's box.
Day BJ. Day BJ. Free Radic Biol Med. 2014 Jan;66:58-64. doi: 10.1016/j.freeradbiomed.2013.05.047. Epub 2013 Jul 12. Free Radic Biol Med. 2014. PMID: 23856377 Free PMC article. Review. - Effects of Hydrogen Peroxide Stress on the Nucleolar Redox Environment and Pre-rRNA Maturation.
Sapio RT, Burns CJ, Pestov DG. Sapio RT, et al. Front Mol Biosci. 2021 Apr 26;8:678488. doi: 10.3389/fmolb.2021.678488. eCollection 2021. Front Mol Biosci. 2021. PMID: 33981726 Free PMC article.
References
- Ligeza A, Tikhonov AN, Hyde JS, Subczynski WK. Biochim Biophys Acta. 1998;1365:453–63. - PubMed
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, et al. Biochem Biophys Res Commun. 1969;36:891–7. - PubMed
- Messner KR, Imlay JA. J Biol Chem. 1999;274:10119–28. - PubMed
- Seaver LC, Imlay JA. J Biol Chem. 2004;279:48742–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM049640/GM/NIGMS NIH HHS/United States
- R37 GM049640/GM/NIGMS NIH HHS/United States
- R37 GM049640-16/GM/NIGMS NIH HHS/United States
- GM 49640/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases