Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation - PubMed (original) (raw)
Review
Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation
Christopher E Cox et al. Crit Care Med. 2008 Mar.
Abstract
Objective: The economic implications of sedative choice in the management of patients receiving mechanical ventilation are unclear because of differences in costs and clinical outcomes associated with specific sedatives. Therefore, we aimed to determine the cost-effectiveness of the most commonly used sedatives prescribed for mechanically ventilated critically ill patients.
Design, setting, and patients: Adopting the perspective of a hospital, we developed a probabilistic decision model to determine whether continuous propofol or intermittent lorazepam was associated with greater value when combined with daily awakenings. We also evaluated the comparative value of continuous midazolam in secondary analyses. We assumed that patients were managed in a medical intensive care unit and expected to require ventilation for > or = 48 hrs. Model inputs were derived from primary analysis of randomized controlled trial data, medical literature, Medicare reimbursement rates, pharmacy databases, and institutional data.
Main results: We measured cost-effectiveness as costs per mechanical ventilator-free day within the first 28 days after intubation. Our base-case probabilistic analysis demonstrated that propofol dominated lorazepam in 91% of simulations and, on average, was both $6,378 less costly per patient and associated with more than three additional mechanical ventilator-free days. The model did not reveal clinically meaningful differences between propofol and midazolam on costs or measures of effectiveness.
Conclusion: Propofol has superior value compared with lorazepam when used for sedation among the critically ill who require mechanical ventilation when used in the setting of daily sedative interruption.
Figures
Figure 1. Decision Model
This is a simplified version of the decision model used in analyses. In this model, patients ventilated for at least 48 hours could either receive propofol or lorazepam for sedation. Progression through the decision tree over the course of the succeeding 28 days examined was determined by probabilities defined in Table 1.
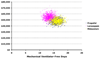
Figure 2. Scatterplot of probabilistic analyses comparing costs and effects
These scatterplots depict the results of 1,000 simulations during which important clinical variables were allowed to vary simultaneously within predefined distributions (see Methods). In contrast to Figure 4, the axes represent the actual (not incremental) costs and effects of each separate trial. This figure demonstrates near duplication of propofol (blue circles) costs and effects for midazolam (yellow circles), though little overlap of lorazepam with either of these groups—emphasizing the dominance of propofol and midazolam over lorazepam (pink circles).
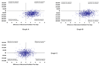
Figure 3. Scatterplot of probabilistic analyses comparing incremental costs and effects
These scatterplots graph the results of 1,000 simulations during which important clinical variables were allowed to vary simultaneously within predefined distributions (see Methods). The x axis represents the incremental difference in mechanical ventilator-free days by group (propofol – lorazepam in Graph A, midazolam – lorazepam in Graph B, and propofol – midazolam in Graph C). The y axis represents incremental costs calculated in a similar fashion. The clustering of simulation results in the lower right quadrant in Graphs A and B demonstrates the comparative dominance of propofol and midazolam over lorazepam. The clustering of analyses at the intersection of the axes in Graph C suggests that there is little evidence that clinically or economically important differences exist between propofol and midazolam.
Figure 4. Tornado diagram comparing cost differences between lorazepam and propofol
The length of the horizontal bars corresponds to the difference in average costs between lorazepam and propofol groups over the range specified for variables of interest depicted on the y axis. The vertical line transecting the bars represents the cost difference between lorazepam and propofol in the point-estimate (non-probabilistic) base-case analysis. The horizontal line at the top of the figure represents the range of values over which propofol’s cost is less than that of lorazepam. There were fewer mechanical ventilator-free days in all ranges and scenarios with the exception of a propofol to lorazepam ratio of mechanical ventilation duration >1.5 (for example, 6 propofol ventilator days to 4 lorazepam ventilator days). The grey bar and horizontal arrow depict this gain in lorazepam-associated mechanical ventilator-free days.
Comment in
- Importance of systems-based practice in achieving pharmacoeconomic benefits.
Lumb PD. Lumb PD. Crit Care Med. 2008 Mar;36(3):990-1. doi: 10.1097/CCM.0B013E3181671137. Crit Care Med. 2008. PMID: 18431293 No abstract available.
Similar articles
- Pharmacoeconomic modeling of lorazepam, midazolam, and propofol for continuous sedation in critically ill patients.
MacLaren R, Sullivan PW. MacLaren R, et al. Pharmacotherapy. 2005 Oct;25(10):1319-28. doi: 10.1592/phco.2005.25.10.1319. Pharmacotherapy. 2005. PMID: 16185175 - A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients.
Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, Bourdet S, Ivanova A, Henderson AG, Pohlman A, Chang L, Rich PB, Hall J. Carson SS, et al. Crit Care Med. 2006 May;34(5):1326-32. doi: 10.1097/01.CCM.0000215513.63207.7F. Crit Care Med. 2006. PMID: 16540958 Clinical Trial. - Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs.
Barrientos-Vega R, Mar Sánchez-Soria M, Morales-García C, Robas-Gómez A, Cuena-Boy R, Ayensa-Rincon A. Barrientos-Vega R, et al. Crit Care Med. 1997 Jan;25(1):33-40. doi: 10.1097/00003246-199701000-00009. Crit Care Med. 1997. PMID: 8989173 Clinical Trial. - Sedation in the intensive care unit.
Young C, Knudsen N, Hilton A, Reves JG. Young C, et al. Crit Care Med. 2000 Mar;28(3):854-66. doi: 10.1097/00003246-200003000-00041. Crit Care Med. 2000. PMID: 10752842 Review. - Analgosedation: a paradigm shift in intensive care unit sedation practice.
Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Devabhakthuni S, et al. Ann Pharmacother. 2012 Apr;46(4):530-40. doi: 10.1345/aph.1Q525. Epub 2012 Apr 10. Ann Pharmacother. 2012. PMID: 22496477 Review.
Cited by
- Anesthetic Propofol-Induced Gene Expression Changes in Patients Undergoing Coronary Artery Bypass Graft Surgery Based on Dynamical Differential Coexpression Network Analysis.
Yu D, Huang LJ, Chen NM. Yu D, et al. Comput Math Methods Med. 2016;2016:7097612. doi: 10.1155/2016/7097612. Epub 2016 Jun 29. Comput Math Methods Med. 2016. PMID: 27437027 Free PMC article. - Propofol Increases Host Susceptibility to Microbial Infection by Reducing Subpopulations of Mature Immune Effector Cells at Sites of Infection.
Visvabharathy L, Xayarath B, Weinberg G, Shilling RA, Freitag NE. Visvabharathy L, et al. PLoS One. 2015 Sep 18;10(9):e0138043. doi: 10.1371/journal.pone.0138043. eCollection 2015. PLoS One. 2015. PMID: 26381144 Free PMC article. - Perioperative dexmedetomidine reduces delirium after coronary artery bypass graft surgery: A prospective, single-blind, observational study.
Singh A, Garg V, Mehta Y, Bhan A, Trehan N. Singh A, et al. Ann Card Anaesth. 2022 Oct-Dec;25(4):490-497. doi: 10.4103/aca.aca_45_21. Ann Card Anaesth. 2022. PMID: 36254916 Free PMC article. - Sedation for critically ill or injured adults in the intensive care unit: a shifting paradigm.
Roberts DJ, Haroon B, Hall RI. Roberts DJ, et al. Drugs. 2012 Oct 1;72(14):1881-916. doi: 10.2165/11636220-000000000-00000. Drugs. 2012. PMID: 22950534 Review. - A cost-effectiveness analysis of propofol versus midazolam for the sedation of adult patients admitted to the intensive care unit.
Andrade TR, Salluh JIF, Garcia R, Farah D, Silva PSLD, Bastos DF, Fonseca MCM. Andrade TR, et al. Rev Bras Ter Intensiva. 2021 Jul-Sep;33(3):428-433. doi: 10.5935/0103-507X.20210068. Rev Bras Ter Intensiva. 2021. PMID: 35107554 Free PMC article.
References
- Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016–1024. - PubMed
- Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest. 2000;118(4):1100–1105. - PubMed
- Rotondi AJ, Chelluri L, Sirio C, et al. Patients' recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752. - PubMed
- Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 GM63906/GM/NIGMS NIH HHS/United States
- K23 HL067068/HL/NHLBI NIH HHS/United States
- HL067068/HL/NHLBI NIH HHS/United States
- K23 HL081048/HL/NHLBI NIH HHS/United States
- K23 HL081048-01/HL/NHLBI NIH HHS/United States
- K23 HL081048-02/HL/NHLBI NIH HHS/United States
- K23 HL081048-04/HL/NHLBI NIH HHS/United States
- K23 HL081048-03/HL/NHLBI NIH HHS/United States
- K23 GM063906/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources