Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state - PubMed (original) (raw)
Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state
Thomas D Marose et al. Dev Biol. 2008.
Abstract
Differentiation is the process by which tissues/organs take on their final, physiologically functional form. This process is mediated in part by the silencing of embryonic genes and the activation of terminal, differentiation gene products. Mammalian kidney development is initiated when the Wolffian duct branches and invades the overlying metanephric mesenchyme. The newly formed epithelial bud, known as the ureteric bud, will continue to branch ultimately differentiating into the collecting duct system and ureter. Here, we show that Hoxb7-Cre mediated removal of beta-catenin from the mouse Wolffian duct epithelium leads to the premature expression of gene products normally associated with the differentiated kidney collecting duct system including the water channel protein, Aquaporin-3 and the tight junction protein isoform, ZO-1 alpha+. Mutant cells fail to maintain expression of some genes associated with embryonic development, including several mediators of branching morphogenesis, which subsequently leads to kidney aplasia or hypoplasia. Reciprocally, expression of a stabilized form of beta-catenin appears to block differentiation of the collecting ducts. All of these defects occur in the absence of any effects on the adherens junctions. These data indicate a role for beta-catenin in maintaining cells of the Wolffian ducts and the duct derived ureteric bud/collecting duct system in an undifferentiated or precursor state.
Figures
Figure 1
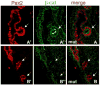
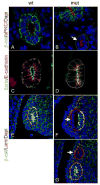
Sagittal sections through e11.5 β–catenin mutant Wolffian ducts. β–catenin staining shows that extruded epithelia can be either wildtype (A) or mutant (B). Ducts have been double stained with antibodies for Pax-2 (single channel red in A′ and B′) and b-catenin (single channel green in A″ and B″).
Figure 2
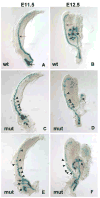
Development of the Wolffian duct and ureteric bud at e11.5 and e12.5. Lac-Z stained e11.5 (A, C, E) and e12.5 (B, D, F) Hoxb7-Cre; β–catenin+/c;ROSA+/− (wildtype, A and B) and Hoxb7-Cre; β–catenin−/c;ROSA+/− (mutant, C–F) urogenital systems. Hoxb7-Cre is active in the Wolffian duct (red arrows) and ureteric bud (black arrows) of e11.5–12.5 urogenital systems (A and B). β-catenin mutant collecting ducts show multiple ectopic bud structures branching from the Wolffian duct (arrowheads in C–F) and defective branching of the ureteric buds (black arrows in C–F).
Figure 3
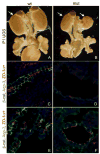
β–catenin mutant phenotypes in P1 kidneys. Wholemount Lac-Z stained (A–E) and H and E stained (F–H) P1 kidneys from Hoxb7-Cre; β–catenin+/c;Rosa+/− (wildtype, A, D and F) and Hoxb7-Cre; β–catenin−/c; Rosa+/− (mutant, B, C, E, G and H) pups. B shows a mutant with duplexed kidneys (blue arrows) and ectopic ureters (black arrows). C shows a severely dysplastic kidney with a single ureter (black arrow). D and E show high magnification images of A and B respectively. Note the dilated collecting ducts in E (black arrow). Sections through wildtype (F) and a cystic (G) and severely dysplastic (H) kidney. Note the expanded renal pelvis in G (asterisk).
Figure 4
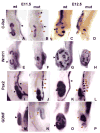
Molecular characterization of β–catenin mutant Wolffian ducts and ureteric buds. Wholemount in situ hybridization of e11.5 (A, B, E, F, I, J, M, N) and 12.5 (C, D, G, H, K, L, O, P) wildtype (A, C, E, G, I, K, M, O) and mutant (B, D, F, H, J, L, N, P) urogenital systems stained with antisense c-Ret (A–D), Wnt11 (E–H), Pax2 (I–L) and GDNF (M–P). In B, the black arrows indicate the wildtype Wolffian duct while the red arrows indicate areas that show no c-Ret expression. Black arrowheads indicate the wildtype metanephros and the red arrowheads indicate the mutant metanephros (ureteric bud in A– H, metanephric mesenchyme in I–P).
Figure 5
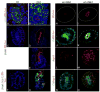
Adherens junctions are not disrupted in β–catenin mutants. Wildtype (A,C,E) and mutant (B,D,E,F,H) stained for pan-cytokeratin (single channel in A′ and B′), E-cadherin (single channel in A″, B″, C″ and D″) and β–catenin (single channel in A‴, B‴, C‴ and D‴) and plakoglobin (C′ and D′) show mutant epithelia (arrows in B) express E-cadherin and plakoglobin normally. Toluidine blue stained sections (E and F) show histology of mutant extruded epithelia (arrowhead in F) relative to wildtype epithelia (E and arrow in F). Transmission electron microscopy showing the adherens junctions (arrows in G and H) are morphologically undisturbed in mutant epithelia (H) relative to wildtype (G). Magnification in G and H is approx. 60,000X).
Figure 6
β-catenin mutant cells show normal apical/basal polarity. Wildtype (A, C and E) and mutant (B, D, F and G) e11.5 Wolffian ducts triple stained for the apical membrane marker aPKC (red), β-catenin (green) and Dapi (blue) in A and B, the baso-lateral membrane marker E-cadherin (red), the apical cytoskeletal marker Ezrin (green) and β-catenin (not shown) in C and D or the basal lamina marker laminin (red), β-catenin (green) and Dapi (blue) in E–G. β–catenin mutant epithelia are indicated by dotted white lines in B and D. A–D are 1000X. E–G are 630X.
Figure 7
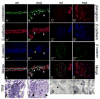
ZO-1α+ expression inversely correlates with high levels of β-catenin activity in the developing WD and ureteric buds. Sections of e11.5 (A), 14.5 (B and F) and P1 (C) Wolffian ducts (A) and kidneys (B–C and F) stained with antibodies to the WD/UB/CD marker cytokeratin (blue), ZO-1α− (red) and ZO-1α+ (green) in A–C or a de-phosphorylated beta-catenin antibody (green in F). ZO-1α+ is not expressed in the e11.5 and 12.5 WD and ureteric bud (arrow in A and data not shown). ZO-1α+ expression is detectable in the e14.5 CD (white arrow in B) but not in the proximal tips of the UB (arrowhead in B). The majority of the UB/CD system is ZO-1α+ positive at P1 (arrows in C). ZO-1α+ is expressed in cells that lack high β-catenin transcriptional activity as indicated by the expression of Axin2 mRNA and excluded from cells with the highest levels of active (de-phosphorylated) β-catenin (arrowhead in F). Axin-2 is expressed throughout the e11.5 Wolffian duct (black arrow in D) and ureteric bud (black arrowhead in D) but only in the ureteric bud tips at e14.5 and P1 (black arrowhead in E and not shown).
Figure 8
Blocking β–catenin function leads to the premature production of differentiation factors. Transverse sections through the Wolffian ducts of wildtype (A, C, F) or mutant (B, D, E, G) embryos. (A–B) Wolffian ducts triple stained for β–catenin (green), ZO-1α+ (red) and Dapi (blue). Note the expression of ZO-1α+ only in the β–catenin mutant epithelia (arrow in B). (C–E) Wolffian ducts stained for ZO-1α+ (red) and β-catenin (blue). Once again, β-catenin is only in the mutant cells, even when they are still part of an intact but mosaic Wolffian duct (E). F–G, Wolffian ducts stained with, aquaporin-3 (green), ZO-1α+ (red) and β–catenin (blue). Note the expression of Aqp-3 in the β–catenin mutant cells but not in wildtype cells from the same duct (arrow in G). β-catenin mutant cells are outlined with dotted line in all images. H –O show transverse sections through e10.5 Wolffian ducts cultures for 48 hours in the presence of 200ng/ml BSA (H, J, L and N) or recombinant Dkk1 (I, K, M and O) stained with ZO-1α+ (Red in H–K. H and I show single channel.), Aqp-3 (Red in L–O. L and M show single channel.) and cytokeratin (green in J, K, N and O). A–G are 1000x, H and I are 630X.
Figure 9
Expression of a stabilized form of β-catenin leads to hypoplasia and a lack of differentiation. Wholemount (A–B) and sections (C–F) of urogenital systems from P1 β-cat ex3fl/+ (A,C,E) or Hoxb7cre; β-cat ex3fl/+ (B,D,F). Sections have been stained with antibodies to Aqp-3 (green), ZO-1α+ (red) and β-catenin (blue) in C–D or Aqp-2 (green), ZO-1α+ (red) and β-catenin (blue) in E–F. Collecting ducts of mice expressing an activated form of β-catenin show greatly decreased levels of Aqp-2 and 3. Arrows in A and B indicate the kidneys.
Similar articles
- Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis.
Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F. Chi X, et al. Dev Cell. 2009 Aug;17(2):199-209. doi: 10.1016/j.devcel.2009.07.013. Dev Cell. 2009. PMID: 19686681 Free PMC article. - Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction.
Grote D, Boualia SK, Souabni A, Merkel C, Chi X, Costantini F, Carroll T, Bouchard M. Grote D, et al. PLoS Genet. 2008 Dec;4(12):e1000316. doi: 10.1371/journal.pgen.1000316. Epub 2008 Dec 26. PLoS Genet. 2008. PMID: 19112489 Free PMC article. - Sall4 Is Transiently Expressed in the Caudal Wolffian Duct and the Ureteric Bud, but Dispensable for Kidney Development.
Toyoda D, Taguchi A, Chiga M, Ohmori T, Nishinakamura R. Toyoda D, et al. PLoS One. 2013 Jun 18;8(6):e68508. doi: 10.1371/journal.pone.0068508. Print 2013. PLoS One. 2013. PMID: 23825698 Free PMC article. - miRNAs in mammalian ureteric bud development.
Yu J. Yu J. Pediatr Nephrol. 2014 Apr;29(4):745-9. doi: 10.1007/s00467-013-2734-y. Epub 2014 Jan 23. Pediatr Nephrol. 2014. PMID: 24452329 Review. - The ureteric bud epithelium: morphogenesis and roles in metanephric kidney patterning.
Nagalakshmi VK, Yu J. Nagalakshmi VK, et al. Mol Reprod Dev. 2015 Mar;82(3):151-66. doi: 10.1002/mrd.22462. Epub 2015 Mar 17. Mol Reprod Dev. 2015. PMID: 25783232 Free PMC article. Review.
Cited by
- Mammalian kidney development: principles, progress, and projections.
Little MH, McMahon AP. Little MH, et al. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a008300. doi: 10.1101/cshperspect.a008300. Cold Spring Harb Perspect Biol. 2012. PMID: 22550230 Free PMC article. Review. - Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract.
Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM. Gimelli S, et al. Hum Mutat. 2010 Dec;31(12):1352-9. doi: 10.1002/humu.21378. Epub 2010 Nov 9. Hum Mutat. 2010. PMID: 20960469 Free PMC article. - The GDNF target Vsnl1 marks the ureteric tip.
Ola R, Jakobson M, Kvist J, Perälä N, Kuure S, Braunewell KH, Bridgewater D, Rosenblum ND, Chilov D, Immonen T, Sainio K, Sariola H. Ola R, et al. J Am Soc Nephrol. 2011 Feb;22(2):274-84. doi: 10.1681/ASN.2010030316. J Am Soc Nephrol. 2011. PMID: 21289216 Free PMC article. - Renal Tubule Repair: Is Wnt/β-Catenin a Friend or Foe?
Gewin LS. Gewin LS. Genes (Basel). 2018 Jan 24;9(2):58. doi: 10.3390/genes9020058. Genes (Basel). 2018. PMID: 29364168 Free PMC article. Review. - From ureteric bud to the first glomeruli: genes, mediators, kidney alterations.
Fanos V, Loddo C, Puddu M, Gerosa C, Fanni D, Ottonello G, Faa G. Fanos V, et al. Int Urol Nephrol. 2015 Jan;47(1):109-16. doi: 10.1007/s11255-014-0784-0. Epub 2014 Sep 9. Int Urol Nephrol. 2015. PMID: 25201458 Review.
References
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–24. - PubMed
- Balda MS, Matter K. Epithelial cell adhesion and the regulation of gene expression. Trends Cell Biol. 2003;13:310–8. - PubMed
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–39. - PubMed
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105 ( Pt 4):1025–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous