Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants - PubMed (original) (raw)
Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants
Jessica Sullivan-Brown et al. Dev Biol. 2008.
Abstract
Zebrafish are an attractive model for studying the earliest cellular defects occurring during renal cyst formation because its kidney (the pronephros) is simple and genes that cause cystic kidney diseases (CKD) in humans, cause pronephric dilations in zebrafish. By comparing phenotypes in three different mutants, locke, swt and kurly, we find that dilations occur prior to 48 hpf in the medial tubules, a location similar to where cysts form in some mammalian diseases. We demonstrate that the first observable phenotypes associated with dilation include cilia motility and luminal remodeling defects. Importantly, we show that some phenotypes common to human CKD, such as an increased number of cells, are secondary consequences of dilation. Despite having differences in cilia motility, locke, swt and kurly share similar cystic phenotypes, suggesting that they function in a common pathway. To begin to understand the molecular mechanisms involved in cyst formation, we have cloned the swt mutation and find that it encodes a novel leucine rich repeat containing protein (LRRC50), which is thought to function in correct dynein assembly in cilia. Finally, we show that knock-down of polycystic kidney disease 2 (pkd2) specifically causes glomerular cysts and does not affect cilia motility, suggesting multiple mechanisms exist for cyst formation.
Figures
Figure 1. locke, swt and kurly mutants develop pronephric cysts
(A–H) 3 dpf, (I–P) 5 dpf. At 3 dpf, locke, swt and kurly mutants are easily identified by their “curly-tail down” phenotype. (E–H) are higher magnification images of (A–D), showing the cystic dilations visible under light microscopy. At 5 dpf, the cystic dilations have increased in size shown in (I–L) and magnified in (M–P). The black arrowheads mark the location of the cysts, posterior to the eye and ear. In general, locke mutants have smaller cysts than swt or kurly.
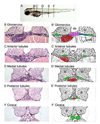
Figure 2. Histological and schematic representations of the pronephros along the anterior to posterior axis
(A) Depiction of the corresponding regions referred to in the text. (B,C,D,E,F) Histological sections stained with Haematoxylin and Eosin. Pictures taken with a 40x objective lens. (B’,C’,D’,E’,F’) Schematic diagrams highlighting regions of interest. (B,B’) Glomerulus: The glomerulus (pink) is found ventral to the notochord (nc) and medial to either somite (s). Connecting to the glomerulus are tubules that extend laterally (blue). The tubules then turn at the edge of the somites and extend towards the posterior (green). Also shown is the gut (red). (C,C’) Anterior tubules: The region designated as the anterior tubules is slightly posterior to the glomerulus region in which the viscera can be observed. (D,D’) Medial tubules: In this region, the gut (red) and tubules (green) are positioned toward the midline and ventral to the notochord (nc). (E,E’) Posterior tubules: The gut has become smaller and the tubules (green) are positioned more medially. (F,F’) Cloaca: This is the most posterior section before the tubules fuse into a single opening outside the body
Figure 3. Temporal and spatial analysis of cystic kidneys in zebrafish mutants
(A–H) 2 dpf; (I–T) 2.5 dpf. At 2 dpf the glomerulus (arrows, A–D) appears intact in the mutant embryos, however the medial tubules have become dilated (arrowheads E–H). At 2.5 dpf, the glomerulus (arrows, I–L) and surrounding Bowman’s space have become enlarged, compressing the podocytes in the mutants. The medial tubules are grossly dilated (arrowheads, M–P). Interestingly, the posterior tubules are less dilated, approaching wildtype size (arrowheads, Q–T). All pictures are 4µm JB-4 plastic sections, stained with Haematoxylin and Eosin and taken with a 100x oil objective lens.
Figure 4. Average cell number surrounding the medial tubules in the mutant backgrounds is increased at 3 dpf
(A) Bar graphs display the average cell number surrounding the glomerular-anterior tubule lumen at 2 dpf and 3 dpf. At 2 dpf there is not a significant increase in cell number surrounding the tubules between wildtype sibling and mutant populations. However, at 3 dpf, there is a statistical difference in cell number in locke and kurly mutant embryos. (B) Bar graphs depict the average cell number surrounding the medial tubule lumen at 2 dpf, 3 dpf and 5 dpf. At 2 dpf, there is not a significant increase in cell number between the wildtype sibling and mutant populations. However at 3 dpf and 5 dpf, the number of cells surrounding the lumen is significantly increased when compared to wildtype siblings. Interestingly, the number of cells in the wildtype sibling population decreases from 2dpf to 5dpf. In all graphs, the error bars represent standard error. Color code: black bars (sibling), striped pattern (locke), crossed pattern (swt), and dark gray (kurly)
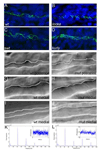
Figure 5. Early pronephric cyst phenotypes
(A–D) Acetylated tubulin staining in the pronephric tubules at 27 hpf. Cilia were detected using an anti-acetylated tubulin antibody (green) and counterstained with the nuclear marker Hoechst (blue). Cilia appeared grossly normal in swt and kurly, but are shorter in locke when compared to wildtype siblings. (E–J) DIC microscopy images of the pronephric tubules in wildtype siblings and kurly mutants. Lumen sizes in posterior tubules from 26–30hpf are similar in both wildtype siblings (E) and mutant embryos (F). Lumens in the medial tubules are larger in both wildtype siblings (G) and mutant embryos (H) when compared in the posterior regions (E,F). By 2 dpf there is a clear dilation in the medial tubules of mutant embryos (J) as compared to wildtype siblings (I). (K,L) Infra-red scattering measurements demonstrate that the frequency of cilia movement in kurly mutants (L) is similar to the frequency observed in wildtype sibling embryos (K) (~42Hz). Both spectra show several pics. The first pic in each spectrum corresponds to the fundamental frequency of cilia movement. The subsequent pic corresponds to the harmonics of the fundamental frequency (~n*42Hz with n = 1,2, and 3). E–J were taken with a 60x objective water immersion lens using two different cameras. E–H were taken with an iXon camera (Andor); pixel size 16*16 µm. I,J were taken with a Luca camera (Andor); pixel size of 10*10 µm. Because of differences in pixel size, luminal size in E–H cannot be directly compared to I,J.
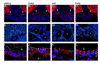
Figure 6. Na+/K+ ATPase localization is disrupted in cystic tissues, while apical polarity is maintained
Apical localization of F-actin (red) observed in wildtype siblings (A) is maintained in the medial tubules (white arrowheads) of mutants (B–D). The somites and gut are also stained by phalloidin (red). Correct apical localization of ZO-1 (green) is observed in wildtype sibling (E) and mutant (F–H) embryos. Sibling tubules are outlined by a white circle, and mutant tubules are distinguished by asterisks. Basolateral localization of the Na+/K+ ATPase (red) observed in wildtype siblings (I) is altered in cystic mutants at 4 dpf (J–L). Note in J–L that Na+/K+ ATPase is adjacent to apical ZO-1 expression (green) which is not observed in wildtype (I). All confocal images were taken with a 40x water objective lens. A–D are cryosections; E–L are plastic sections. Nuclei (blue) are stained with either DRAQ5 (A–D) or Hoechst (E–L). ZO-1 staining basal to the pronephros comes from a different tissue (asterisks in K and L) and does not reflect an alteration in ZO-1 within the pronephros.
Figure 7. swt encodes lrrc50, a novel gene required for cilia motility
(A) Schematic diagram of the LRRC50 protein highlighting the location of tm317 and fk03a mutations. LRRC50 contains 6 predicted leucine rich repeats (cylinders), followed by a coiled-coil domain (spiral), however the functions of these motifs in Swt are unknown. (B). Sequence traces of wildtype sibling and mutant DNA, showing the mutations in each allele. The swttm317 allele creates a missense mutation changing AAC (ASN) to AAA (LYS) at amino acid position 172 in the leucine rich repeat region. The swtfk03a allele creates a nonsense mutation changing GAG (GLU) to TAG (stop codon) at amino acid position 259 after the leucine rich repeats before the coiled-coil domain. swt RNA is expressed in cells that contain cilia: (C) Kupffer’s vesicle (arrow); (D) neural tube staining (arrowhead) and bilateral intermediate mesoderm staining (arrows); (E) otic vesicle (arrow); (F) cross section showing expression in the floorplate of the neural tube (arrowhead); (G) lateral view of otic vesicle expression (arrow); (H) pronephric duct (arrow, dorsal to the yolk extension) and chordo-neural hinge expression (arrowhead, at the tip of the tail). Embryo in C is at tailbud stage (dorsal view of posterior), D–G is at the 12 somite stage, and H is at 24hpf. In D, E anterior is up.
Figure 8. pkd2 morphants develop glomerular dilations, but do not become dilated in the tubular region of the nephron
(A–D) Glomerular region comparison at 3 dpf of a wildtype sibling embryo (A), pkd2 morphants (B,C), and a locke mutant embryo (D). Black arrows mark the glomerulus, which is clearly dilated in both pkd2 morphants and locke mutant embryos. (E–H) Anterior tubule region, immediately posterior to the glomerulus; although the tubules exhibit dilation in locke mutant embryos (H), the tubules in pkd2 morphants (F,G) are not enlarged and resemble wildtype sibling tubules (E). (I–L) Medial tubule region; unlike locke mutant embryos (L), the lumens of the medial tubules are not dilated in pkd2 morphants (J,K), similar to wildtype siblings (I). Black arrowheads indicate tubules. All pictures are from JB-4 plastic sections, stained with an H&E dye, and taken with 40x lens (A–H) and 100x oil lens (I–L).
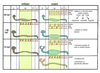
Figure 9. Formation of pronephric cysts in zebrafish mutant embryos
In this model, longitudinal views of the pronephros along the anterior-posterior axis (left to right) are depicted and labeled. The 5 regions of interest are G=glomerulus, A=anterior tubule, M=medial tubule, P=posterior tubule, C=cloaca. At 30 hpf, the nephron of both wildtype siblings (gray) and mutant (blue) embryos appear similar. At this stage, the cilia (red lines) in wildtype embryos are motile, but do not bundle. The mutant embryos each exhibit their own unique cilia motility defects at this stage. At 48 hpf, cells in the wildtype sibling medial tubules are multi-ciliated and the cilia have begun to bundle and move in a coordinated fashion. Cells in the posterior and cloaca regions contain monocilia which are motile as well. It is at the medial tubules where the cystic dilations begin in the mutant embryos. Although the medial tubules are drastically dilated in mutants, the lumens in the posterior and cloaca regions are similar in size to wildtype siblings. At 72hpf, the medial tubules in the mutant embryos continue to dilate, while the wildtype sibling medial tubules decrease in size. At this stage, the anterior tubule and glomerular region of the mutant embryos are affected. The pkd2 morphants (yellow) display cystic dilations specifically in the glomerulus. The medial tubule in pkd2 morphants does not dilate and shows normal cilia motility. The highlighted column depicts the medial tubules.
Similar articles
- Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function.
Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Drummond IA, et al. Development. 1998 Dec;125(23):4655-67. doi: 10.1242/dev.125.23.4655. Development. 1998. PMID: 9806915 - Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning.
Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Serluca FC, et al. Development. 2009 May;136(10):1621-31. doi: 10.1242/dev.020735. Development. 2009. PMID: 19395640 Free PMC article. - A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney.
Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. Sun Z, et al. Development. 2004 Aug;131(16):4085-93. doi: 10.1242/dev.01240. Epub 2004 Jul 21. Development. 2004. PMID: 15269167 - The zebrafish pronephros: a genetic system for studies of kidney development.
Drummond IA. Drummond IA. Pediatr Nephrol. 2000 May;14(5):428-35. doi: 10.1007/s004670050788. Pediatr Nephrol. 2000. PMID: 10805474 Review. - Imaging cilia in zebrafish.
Jaffe KM, Thiberge SY, Bisher ME, Burdine RD. Jaffe KM, et al. Methods Cell Biol. 2010;97:415-35. doi: 10.1016/S0091-679X(10)97022-2. Methods Cell Biol. 2010. PMID: 20719283 Review.
Cited by
- An Overview of In Vivo and In Vitro Models for Autosomal Dominant Polycystic Kidney Disease: A Journey from 3D-Cysts to Mini-Pigs.
Koslowski S, Latapy C, Auvray P, Blondel M, Meijer L. Koslowski S, et al. Int J Mol Sci. 2020 Jun 25;21(12):4537. doi: 10.3390/ijms21124537. Int J Mol Sci. 2020. PMID: 32630605 Free PMC article. Review. - Antennas of organ morphogenesis: the roles of cilia in vertebrate kidney development.
Marra AN, Li Y, Wingert RA. Marra AN, et al. Genesis. 2016 Sep;54(9):457-69. doi: 10.1002/dvg.22957. Epub 2016 Jul 25. Genesis. 2016. PMID: 27389733 Free PMC article. Review. - Cilia in the developing zebrafish ear.
Whitfield TT. Whitfield TT. Philos Trans R Soc Lond B Biol Sci. 2020 Feb 17;375(1792):20190163. doi: 10.1098/rstb.2019.0163. Epub 2019 Dec 30. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31884918 Free PMC article. Review. - The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation.
Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, McSheene J, Loges NT, Olbrich H, Haeffner K, Fliegauf M, Horvath J, Reinhardt R, Nielsen KG, Marthin JK, Baktai G, Anderson KV, Geisler R, Niswander L, Omran H, Burdine RD. Becker-Heck A, et al. Nat Genet. 2011 Jan;43(1):79-84. doi: 10.1038/ng.727. Epub 2010 Dec 5. Nat Genet. 2011. PMID: 21131974 Free PMC article. - c21orf59/kurly Controls Both Cilia Motility and Polarization.
Jaffe KM, Grimes DT, Schottenfeld-Roames J, Werner ME, Ku TS, Kim SK, Pelliccia JL, Morante NF, Mitchell BJ, Burdine RD. Jaffe KM, et al. Cell Rep. 2016 Mar 1;14(8):1841-9. doi: 10.1016/j.celrep.2016.01.069. Epub 2016 Feb 18. Cell Rep. 2016. PMID: 26904945 Free PMC article.
References
- The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;78:725. - PubMed
- The International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. - PubMed
- Badano JL, et al. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. - PubMed
- Baert L. Hereditary polycystic kidney disease (adult form): a microdissection study of two cases at an early stage of the disease. Kidney Int. 1978;13:519–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01HD048584/HD/NICHD NIH HHS/United States
- P50GM071508/GM/NIGMS NIH HHS/United States
- P50 GM071508/GM/NIGMS NIH HHS/United States
- R01 HD048584/HD/NICHD NIH HHS/United States
- R01 HD048584-01A1/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous