PK11195 labels activated microglia in Alzheimer's disease and in vivo in a mouse model using PET - PubMed (original) (raw)
PK11195 labels activated microglia in Alzheimer's disease and in vivo in a mouse model using PET
Sriram Venneti et al. Neurobiol Aging. 2009 Aug.
Abstract
Activated microglia may promote neurodegeneration in Alzheimer's disease (AD) and may also help in amyloid clearance in immunization therapies. In vivo imaging of activated microglia using positron emission tomography (PET) could assist in defining the role of activated microglia during AD progression and therapeutics. We hypothesized that PK11195, a ligand that binds activated microglia, could label these cells in postmortem AD tissues and in vivo in an animal model of AD using PET. [(3)H](R)-PK11195 binding was significantly higher in AD frontal cortex compared to controls and correlated mainly with the abundance of immunohistochemically labeled activated microglia. With age, the brains of APP/PS1 transgenic mice showed progressive increase in [(3)H](R)-PK11195 binding and [(11)C](R)-PK11195 retention in vivo assessed using microPET, which correlated with the histopathological abundance of activated microglia. These results suggest that PK11195 binding in AD postmortem tissue and transgenic mice in vivo correlates with the extent of microglial activation and may help define the role of activated microglia in the pathogenesis and treatment of AD.
Conflict of interest statement
Disclosure statement: All authors declare no proprietary interest or any conflict of interest related to this study.
Figures
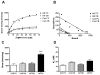
Figure 1. [3H](R)-PK11195 is higher in the AD frontal cortex
Filtration binding with [3H](R)-PK11195 was assessed in the frontal cortex (FC) and cerebellum (CB) of AD cases (n=5) and controls (Con) (n=6). (A & B) Representative curves (A) and scatchard plots (B) from the FC (black squares) of AD showed significantly higher specific binding (per mg protein) than brain tissue obtained from the CB of AD cases (gray squares), FC (black open squares) and CB (gray open squares) brain tissue obtained from controls. (C) Bmax (reflective of the total number of binding sites) with [3H](R)-PK11195 was significantly higher in AD FC (black bars) compared with AD CB (gray bars), FC (clear bars) and CB (hatched bars) tissue obtained from controls (_p_=0.0002). (D) The KD (reflective of the binding affinity) was not significantly different in all the conditions (_p_=0.3187). Data was analyzed using one-way ANOVA with 95% confidence intervals.
Figure 2. [3H](R)-PK11195 increases in Tg mice with age
Filtration binding with [3H](R)-PK11195 was assessed in the frontal cortex (FC) of Tg mice and wild type controls (Con) in the age groups 13–16 and 16–19 months (n=3, each). (A & B) Representative curves (A) and scatchard plots (B) of Tg mice in the age groups 16–19 months (black squares) showed significantly higher specific binding (per mg protein) than Tg mice in the age group 13–16 months (gray squares) and both control (black and gray squares) groups. (C) Bmax (reflective of the total number of binding sites) with [3H](R)-PK11195 was significantly higher in Tg mice in the age groups 16–19 months (black squares) than Tg mice in the age group 13–16 months (gray bars), and both control groups (clear and hatched bars) (_p_=0.0023). (D) The KD (reflective of the binding affinity) was not significantly different in all the conditions (_p_=0.7249). Data was analyzed using one-way ANOVA with 95% confidence intervals.
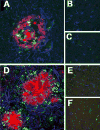
Figure 3. Immunohistochemical evaluation of activated microglia and reactive astrocytosis in AD and Tg mice
(A–C) Frontal tissue from AD in regions containing plaques (A) showed increased staining for microglia (CD68, green), astrocytes (GFAP, blue) and Aβ (red) compared to plaque free areas in the same cases (B) and controls (C). (D–F) Frontal tissue from APP/PS1 mice showed increased staining for microglia (Iba-1, green), astrocytes (GFAP, blue) and Aβ (red) in regions containing plaques (D) compared to plaque free areas in the same mice (E) and control, wild type mice (F).
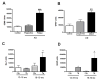
Figure 4. Quantification of activated microglia and reactive astrocytosis in AD and Tg mice
(A & B) Microglia stained with CD68 (A) and astrocytes with GFAP (B) were quantified in AD frontal cortical tissue (n=5, each). Regions containing plaques had significantly higher microglia (A, black bars, p < 0.0001) and astrocytes (B, black bars, p = 0.0003) than regions without plaques (A & B, grey bars) and controls (A & B, clear bars). (C & D) Microglia stained with Iba-1 (C) and astrocytes stained with GFAP (D) were quantified in APP/PS1 (Tg) and wild type control (Con) mice in two age groups: 13–16, and 16–19 months. Regions containing plaques had significantly higher microglia (C, black bars) in Tg (n=5) compared to controls (n=3) (D, hatched bars) mice in the 16–19 months age group (p = 0.048), but not in Tg (n=4) and control (n=3) mice in the 13–16 month age groups (C, clear and grey bars, p = 0.6383). GFAP labeled astrocytes were significantly higher in Tg mice compared to controls in both 13–16 (D, clear and grey bars, p = 0.0416) and 16–19 (D, hatched and black bars, p = 0.0224). Data was analyzed using ANOVA with 95% confidence intervals, ***p<0.001, **p<0.01, *p<0.05.
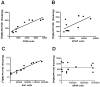
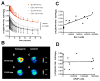
Figure 5. [3H](R)-PK11195 binding corrleates with activated microglia in AD and Tg brain tissues
(A & B) [3H](R)-PK11195 Bmax (Y-axis, fmols/mg) values derived from AD and control cases correlated with the abundance of CD68 labeled activated microglia (A, X-axis, CD68 units) assessed in the same area in these same cases (r = 0.9393, p = 0.0002). [3H](R)-PK11195 Bmax values (Y-axis, fmols/mg) correlated weakly with the extent of reactive astrocytosis (B, X-axis, GFAP units, r = 0.6729, p = 0.0390). (C & D) [3H](R)-PK11195 Bmax (Y-axis, fmols/mg) values derived from Tg and controls correlated with the abundance of Iba-1 labeled activated microglia (C, X-axis, Iba-1 units) assessed in the same area in these same animals (r = 0.9657, p = 0.0007). [3H](R)-PK11195 Bmax values (Y-axis, fmols/mg) did not correlated with the extent of reactive astrocytosis (D, X-axis, GFAP units, r = -0.3203, p = 0.5360).
Figure 6. PET imaging with [11C](R)-PK11195 shows an age-dependent increase in APP/PS1 (Tg) mice, but not controls
(A) APP/PS1 (Tg) and wild type control (Con) mice in the age groups 13–16 and 16–19 months were imaged with microPET using [11C](R)-PK11195. Retention of the radioligand in the brain is represented as radioactivity concentrations summed over the scan frames and normalized to both the injected dose of [11C](R)-PK11195 and the body mass of the animal (%ID/g*kg, Y-axis) and is plotted against time in minutes (X-axis). [11C](R)-PK11195 retention was not different between Tg (n=4, grey squares) and Con (n=3, clear squares) in the 13–16 month age group, but was higher in Tg mice 16–19 month age group (n=5, red squares) compared to all the other animal groups (_p_=0.001). Data was analyzed using one-way ANOVA with 95% confidence intervals. (B) Representative coronal PET images from 16–19 month animals (Tg, and control) and 13–16 month animals (Tg, and control) showing increased [11C](R)-PK11195 retention in the 16–19 month Tg compared to all the other animal groups. (C and D) [11C](R)-PK11195 binding values (%ID/g*kg, Y-axis) derived from Tg and controls correlated with the abundance of Iba-1 labeled activated microglia (C, X-axis, Iba-1 units) assessed in the same area in these same animals (r = 0.7690, p = 0.0433). [11C](R)-PK11195 binding values (%ID/g*kg, Y-axis) did not correlated with the extent of reactive astrocytosis (D, X-axis, GFAP units, r = 0.4100, p = 0.3610)
Similar articles
- Imaging microglial activation and glucose consumption in a mouse model of Alzheimer's disease.
Rapic S, Backes H, Viel T, Kummer MP, Monfared P, Neumaier B, Vollmar S, Hoehn M, Van der Linden A, Heneka MT, Jacobs AH. Rapic S, et al. Neurobiol Aging. 2013 Jan;34(1):351-4. doi: 10.1016/j.neurobiolaging.2012.04.016. Epub 2012 May 30. Neurobiol Aging. 2013. PMID: 22651996 - Detection of Cyclooxygenase-1 in Activated Microglia During Amyloid Plaque Progression: PET Studies in Alzheimer's Disease Model Mice.
Shukuri M, Mawatari A, Ohno M, Suzuki M, Doi H, Watanabe Y, Onoe H. Shukuri M, et al. J Nucl Med. 2016 Feb;57(2):291-6. doi: 10.2967/jnumed.115.166116. Epub 2015 Nov 19. J Nucl Med. 2016. PMID: 26585055 - Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease.
Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST, Mathis CA. Wiley CA, et al. Arch Neurol. 2009 Jan;66(1):60-7. doi: 10.1001/archneurol.2008.511. Arch Neurol. 2009. PMID: 19139300 Free PMC article. - Positron emission tomography imaging of neuroinflammation.
Cagnin A, Kassiou M, Meikle SR, Banati RB. Cagnin A, et al. Neurotherapeutics. 2007 Jul;4(3):443-52. doi: 10.1016/j.nurt.2007.04.006. Neurotherapeutics. 2007. PMID: 17599710 Free PMC article. Review. - [Imaging of brain microgliosis by PET].
Ouchi Y. Ouchi Y. Rinsho Shinkeigaku. 2009 Nov;49(11):925-8. doi: 10.5692/clinicalneurol.49.925. Rinsho Shinkeigaku. 2009. PMID: 20030250 Review. Japanese.
Cited by
- The Role of Glia in Wilson's Disease: Clinical, Neuroimaging, Neuropathological and Molecular Perspectives.
Gromadzka G, Wilkaniec A, Tarnacka B, Hadrian K, Bendykowska M, Przybyłkowski A, Litwin T. Gromadzka G, et al. Int J Mol Sci. 2024 Jul 9;25(14):7545. doi: 10.3390/ijms25147545. Int J Mol Sci. 2024. PMID: 39062788 Free PMC article. Review. - Shining a Light on Venom-Peptide Receptors: Venom Peptides as Targeted Agents for In Vivo Molecular Imaging.
Chow CY, King GF. Chow CY, et al. Toxins (Basel). 2024 Jul 4;16(7):307. doi: 10.3390/toxins16070307. Toxins (Basel). 2024. PMID: 39057947 Free PMC article. Review. - Relationship Between Reactive Astrocytes, by [18F]SMBT-1 Imaging, with Amyloid-Beta, Tau, Glucose Metabolism, and TSPO in Mouse Models of Alzheimer's Disease.
Kong Y, Maschio CA, Shi X, Xie F, Zuo C, Konietzko U, Shi K, Rominger A, Xiao J, Huang Q, Nitsch RM, Guan Y, Ni R. Kong Y, et al. Mol Neurobiol. 2024 Oct;61(10):8387-8401. doi: 10.1007/s12035-024-04106-7. Epub 2024 Mar 19. Mol Neurobiol. 2024. PMID: 38502413 Free PMC article. - APOE ε4 gene dose effect on imaging and blood biomarkers of neuroinflammation and beta-amyloid in cognitively unimpaired elderly.
Snellman A, Ekblad LL, Tuisku J, Koivumäki M, Ashton NJ, Lantero-Rodriguez J, Karikari TK, Helin S, Bucci M, Löyttyniemi E, Parkkola R, Karrasch M, Schöll M, Zetterberg H, Blennow K, Rinne JO. Snellman A, et al. Alzheimers Res Ther. 2023 Apr 4;15(1):71. doi: 10.1186/s13195-023-01209-6. Alzheimers Res Ther. 2023. PMID: 37016464 Free PMC article. - [64Cu]Cu-ATSM: an emerging theranostic agent for cancer and neuroinflammation.
Xie F, Wei W. Xie F, et al. Eur J Nucl Med Mol Imaging. 2022 Oct;49(12):3964-3972. doi: 10.1007/s00259-022-05887-6. Eur J Nucl Med Mol Imaging. 2022. PMID: 35918492 No abstract available.
References
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003;289:2819–2826. - PubMed
- Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer’s disease. Microsc Res Tech. 2001;54:59–70. - PubMed
- Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. - PubMed
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–2337. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 MH001717/MH/NIMH NIH HHS/United States
- R01 MH071151/MH/NIMH NIH HHS/United States
- K24 MH01717/MH/NIMH NIH HHS/United States
- R01 MH064921/MH/NIMH NIH HHS/United States
- R01 MH64921/MH/NIMH NIH HHS/United States
- R01 MH071151-05/MH/NIMH NIH HHS/United States
- R21 AG025829/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- K24 MH001717-10/MH/NIMH NIH HHS/United States
- R21 AG025829-02/AG/NIA NIH HHS/United States
- R01 AG020226/AG/NIA NIH HHS/United States
- P50 AG05133-21/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical