Role of ABO secretor status in mucosal innate immunity and H. pylori infection - PubMed (original) (raw)
Role of ABO secretor status in mucosal innate immunity and H. pylori infection
Sara Lindén et al. PLoS Pathog. 2008 Jan.
Abstract
The fucosylated ABH antigens, which constitute the molecular basis for the ABO blood group system, are also expressed in salivary secretions and gastrointestinal epithelia in individuals of positive secretor status; however, the biological function of the ABO blood group system is unknown. Gastric mucosa biopsies of 41 Rhesus monkeys originating from Southern Asia were analyzed by immunohistochemistry. A majority of these animals were found to be of blood group B and weak-secretor phenotype (i.e., expressing both Lewis a and Lewis b antigens), which are also common in South Asian human populations. A selected group of ten monkeys was inoculated with Helicobacter pylori and studied for changes in gastric mucosal glycosylation during a 10-month period. We observed a loss in mucosal fucosylation and concurrent induction and time-dependent dynamics in gastric mucosal sialylation (carbohydrate marker of inflammation), which affect H. pylori adhesion targets and thus modulate host-bacterial interactions. Of particular relevance, gastric mucosal density of H. pylori, gastritis, and sialylation were all higher in secretor individuals compared to weak-secretors, the latter being apparently "protected." These results demonstrate that the secretor status plays an intrinsic role in resistance to H. pylori infection and suggest that the fucosylated secretor ABH antigens constitute interactive members of the human and primate mucosal innate immune system.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Fucosylated and Sialylated Blood Group (bg) Antigens and Associated Secretor Phenotypes
(A) The α1.2-fucosylated (in red) H and Leb antigens define bg O. Bg A and B antigens present additional GalNAc or Gal residues (blue), respectively. Ley and Leb are both difucosylated. SLea and sLex are sialylated Lewis antigens (in pink). (B) Synthesis pathways for bg antigens with corresponding Se phenotypes: Lea is found in Se0 individuals, whereas Sew individuals carry a mix of mucosal Lea and Leb. Lea is formed when the Se-transferase is inactive or weak, because Lea is a “dead-end” and is not extended further. During inflammation and infection, sialyl-transferases are expressed and carbohydrate core chains become sialylated in competition with Se-fucosyltransferase. (C) The presence of ABH and Lea antigens in salivary, milk, and GI tract secretions identifies individuals of Se, Se0, or Sew phenotype. (D) In Sew subjects, α1.2fucosylation is hampered by an enzymatically weak Se-transferase, whereas Se0 individuals lack Se-transferase activity.
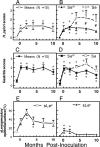
Figure 2. Infection Density, Gastritis, and Mucosal Sialylation in Proximal Stomach (Antrum), in Response to H. pylori Infection (Means ± SEM)
The figure illustrates the time course of H. pylori infection density scores in biopsies from the ten monkeys (A) and in three Se and seven Sew individuals following inoculation (B). Also illustrated is the time course of gastritis scores in biopsies from the ten monkeys (C) and from the three Se and seven Sew individuals following inoculation (D). Finally, the mean percentages of sLea positive (E) and sLex positive surface epithelium (F) are shown in the ten monkeys following inoculation. * Illustrates significant (P <0.05) difference from pre-inoculation value and # illustrates significant (P <0.05) difference between Se and Sew.
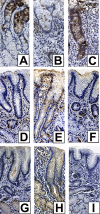
Figure 3. Dynamic Reciprocity in Expression of Fucosylated and Sialylated Antigens in Gastric Mucosa during H. pylori Infection
Leb expression in monkey 8PZ at pre-inoculation (A), 1 week post-inoculation (B), and 2 months post inoculation (C). Thus, gastric mucosal fucosylation was initially strong (A), decreased from 1 to 4 weeks post-inoculation (B), returned to basal level at 2 months (C), and stayed at basal level until 10 months (not shown). The figure also illustrates the expression of sialyl-Lea (D, E, and F) and sialyl-Lex (G, H, and I) in monkey 8PZ at pre-inoculation (D and G), 2 months (E and H), and 10 months post-inoculation (F and I). Thus, gastric mucosal sialylation increased during early infection (not shown), peaked at 2 months (E and H), and returned to pre-inoculation levels at 10 months post-inoculation (F and I).
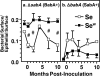
Figure 4. Time Course of Gastric Mucosal Fucosylation and Sialylation following Inoculation of H. pylori to Rhesus Monkeys as Determined by the In Vitro Adherence Assay
The figure illustrates that inoculation of H. pylori induces a strong, time-dependent, increase in mucosal fucosylation and a concurrent suppression of inflammation-associated sialylation in Se monkeys (□), whereas changes are milder in individuals of Sew phenotype (). The changes in the glycosylation pattern were followed by use of in vitro adherence analyses and fluorescent H. pylori bacterial cells as glycosylation-specific lectin tools. Because the H. pylori Δ_sabA_ (BabA+) and Δ_babA_ (SabA+) mutants bind primarily to fucosylated and sialylated structures, respectively, the time-dependent changes measured in terms of in vitro adherence reflect parallel changes in surface epithelium fucosylation and sialylation. In vitro adherence mediated by the BabA adhesin (in Figure S1) identifies the mucosal expression patterns of secretor-dependent fucosylation of ABH and Leb antigens (A), whereas adherence mediated by the SabA adhesin reveals mucosal sialylation (B). Importantly, only in vitro adherence to surface epithelium is shown, because it is fucosylated by the Se-transferase. From each of 120 biopsies, ten gastric pit regions were acquired for digital analysis (i.e., a total of 1,200 mucosal zones were analyzed) [31]. # Illustrates significant (P <0.05) differences between Se and Sew subjects at the corresponding times.
Similar articles
- Blood groups Lewis(b) and ABH expression in gastric mucosa: lack of inter-relation with Helicobacter pylori colonisation and occurrence of gastric MALT lymphoma.
Oberhuber G, Kranz A, Dejaco C, Dragosics B, Mosberger I, Mayr W, Radaszkiewicz T. Oberhuber G, et al. Gut. 1997 Jul;41(1):37-42. doi: 10.1136/gut.41.1.37. Gut. 1997. PMID: 9274469 Free PMC article. - Interrelation between ABH blood group 0, Lewis(B) blood group antigen, Helicobacter pylori infection, and occurrence of peptic ulcer.
Keller R, Dinkel KC, Christl SU, Fischbach W. Keller R, et al. Z Gastroenterol. 2002 May;40(5):273-6. doi: 10.1055/s-2002-30115. Z Gastroenterol. 2002. PMID: 12016560 - Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response.
Heneghan MA, Moran AP, Feeley KM, Egan EL, Goulding J, Connolly CE, McCarthy CF. Heneghan MA, et al. FEMS Immunol Med Microbiol. 1998 Apr;20(4):257-66. doi: 10.1111/j.1574-695X.1998.tb01135.x. FEMS Immunol Med Microbiol. 1998. PMID: 9626930 - Histo-blood group carbohydrates as facilitators for infection by Helicobacter pylori.
Brandão de Mattos CC, de Mattos LC. Brandão de Mattos CC, et al. Infect Genet Evol. 2017 Sep;53:167-174. doi: 10.1016/j.meegid.2017.05.025. Epub 2017 May 31. Infect Genet Evol. 2017. PMID: 28577915 Review. - The ABO, Hh, secretor, and Lewis systems.
Marcus DM. Marcus DM. Immunol Ser. 1989;43:685-99. Immunol Ser. 1989. PMID: 2490511 Review. No abstract available.
Cited by
- In vitro fish mucosal surfaces producing mucin as a model for studying host-pathogen interactions.
Quintana-Hayashi MP, Thomsson Hulthe KA, Lindén SK. Quintana-Hayashi MP, et al. PLoS One. 2024 Aug 9;19(8):e0308609. doi: 10.1371/journal.pone.0308609. eCollection 2024. PLoS One. 2024. PMID: 39121037 Free PMC article. - Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa.
Navabi N, Johansson ME, Raghavan S, Lindén SK. Navabi N, et al. Infect Immun. 2013 Mar;81(3):829-37. doi: 10.1128/IAI.01000-12. Epub 2012 Dec 28. Infect Immun. 2013. PMID: 23275091 Free PMC article. - The Caenorhabditis elegans bus-2 mutant reveals a new class of O-glycans affecting bacterial resistance.
Palaima E, Leymarie N, Stroud D, Mizanur RM, Hodgkin J, Gravato-Nobre MJ, Costello CE, Cipollo JF. Palaima E, et al. J Biol Chem. 2010 Jun 4;285(23):17662-72. doi: 10.1074/jbc.M109.065433. Epub 2010 Apr 12. J Biol Chem. 2010. PMID: 20385555 Free PMC article. - Genomics of host-pathogen interactions: challenges and opportunities across ecological and spatiotemporal scales.
Näpflin K, O'Connor EA, Becks L, Bensch S, Ellis VA, Hafer-Hahmann N, Harding KC, Lindén SK, Olsen MT, Roved J, Sackton TB, Shultz AJ, Venkatakrishnan V, Videvall E, Westerdahl H, Winternitz JC, Edwards SV. Näpflin K, et al. PeerJ. 2019 Nov 5;7:e8013. doi: 10.7717/peerj.8013. eCollection 2019. PeerJ. 2019. PMID: 31720122 Free PMC article. - Both diet and Helicobacter pylori infection contribute to atherosclerosis in pre- and postmenopausal cynomolgus monkeys.
Testerman TL, Semino-Mora C, Cann JA, Qiang B, Peña EA, Liu H, Olsen CH, Chen H, Appt SE, Kaplan JR, Register TC, Merrell DS, Dubois A. Testerman TL, et al. PLoS One. 2019 Sep 6;14(9):e0222001. doi: 10.1371/journal.pone.0222001. eCollection 2019. PLoS One. 2019. PMID: 31490998 Free PMC article.
References
- Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. - PubMed
- Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. - PubMed
- Lindén S, Nordman H, Hedenbro J, Hurtig M, Boren T, et al. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology. 2002;123:1923–1930. - PubMed
- Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical