Kinetochore-microtubule interactions: the means to the end - PubMed (original) (raw)
Review
Kinetochore-microtubule interactions: the means to the end
Tomoyuki U Tanaka et al. Curr Opin Cell Biol. 2008 Feb.
Abstract
Kinetochores are proteinaceous complexes containing dozens of components; they are assembled at centromeric DNA regions and provide the major microtubule attachment site on chromosomes during cell division. Recent studies have defined the kinetochore components comprising the direct interface with spindle microtubules, primarily through structural and functional analysis of the Ndc80 and Dam1 complexes. These studies have facilitated our understanding of how kinetochores remain attached to the end of dynamic microtubules and how proper orientation of a kinetochore-microtubule attachment is promoted on the mitotic spindle. In this article, we review these recent studies and summarize their key findings.
Figures
Figure 1. Overview of kinetochore-microtubule interactions
The figure depicts kinetochore-microtubule interactions during prometaphase (steps 1–3), metaphase (step 4) and anaphase A (step 5). 1) Kinetochores are initially captured by the lateral surface of single microtubules that extend from one of the spindle poles [–8]. The initial kinetochore encounter with microtubules happens quickly following nuclear envelope breakdown (metazoan cells) [6,7] or once kinetochore assembly is complete (budding yeast: note that spindle poles of this organism have not yet separated in step 1–2) [53]. 2) Once captured, kinetochores are transported along the lateral surface of single microtubules toward the spindle pole (sliding) [–8]. Subsequently, at least in budding yeast, kinetochores are tethered at the end of the single microtubules and transported further as the microtubules shrink (end-coupled pulling) [40,53]. 3) As kinetochores approach spindle poles, both sister kinetochores attach to microtubules. If both kinetochores attach to microtubules from the same spindle pole, kinetochore-spindle pole connections by microtubules are re-oriented until proper bi-orientation is established [5,9]. 4) Cessation of re-orientation is dependent on the tension that is generated by microtubules upon establishment of bi-orientation [5,9]. The number of microtubules whose plus ends attach to a single kinetochore increases when tension is applied in metazoan cells [107], while only a single microtubule is thought to attach to a each kinetochore in budding yeast [62] (the latter case is shown here for simplicity). 5) Once all kinetochores bi-orient on the spindle, cohesion between sister chromatids is removed, causing sister chromatid segregation to opposite spindle poles during anaphase A [11]. Kinetochores are end-coupled and pulled poleward as the microtubules depolymerize [12,13].
Figure 2. The Ndc80 csomplex and the KMN network
A) Schematic of the 4-subunit Ndc80 complex indicating the constituent parts and defined domains. Panels on the right show the rod-like structure of the complex visualized by electron microscopy of individual rotary-shadowed recombinant complexes (scale bar 100 nm; reprinted from ref. [23]). The ribbon diagrams below show the calponin-homology domain of the Ndc80 subunit (residues 81–196 of human Ndc80, also known as Hec1) and comparison with the EB1 CH domain (reprinted from [31]). B) Microtubule-binding of the Ndc80 complex. The Ndc80 complex binds to the microtubule lattice with a fixed orientation forming “barbs” that extend away from the lattice. The microtubule-binding activity is located in the globular region of the Ndc80/Nuf2 dimer and is severely reduced by removal of the N-terminal extension on Ndc80. The “barbs” have a uniform polarity and binding angle indicating a specific binding site on the lattice (scale bar 200 nm; reprinted from [30]). C) Schematic of the KNL-1/Mis12 complex/Ndc80 complex (KMN) complex network. Direct association of the Ndc80 complex with these other two kinetochore constituents is conserved in fungi, nematodes, insects, and vertebrates [3]. The Spc24/25 dimer is required for association with KNL-1 and the Mis12 complex. KNL-1 also directly binds to microtubules. Aurora B negatively regulates the microtubule-binding activity of the Ndc80 complex by phosphorylating the basic N-terminal extension of the Ndc80 subunit [30].
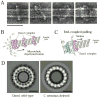
Figure 3. Dam1 complexes oligomerize to form a ring encircling a microtubule
A) Electron micrographs of negatively stained microtubules in the presence of the Dam1 complexes. Scale bar, 50 nm. Reprinted from [44]. B) If the Dam1 complexes formed rings around microtubules, they would be accumulated at the microtubule plus end during the outward curling of protofilaments that accompanies depolymerization; this accumulation was observed in vitro [46] and in vivo [40]. C) The Dam1 complex has a crucial role in tethering kinetochores at microtubule ends and in converting microtubule depolymerization into kinetochore pulling force [40,46,49]. D) Electron micrographs of rings (negative stain and 16-fold rotational average), formed by the Dam1 complexes containing either a wild-type Dam1 protein (left) or a C-terminus (140 residues)-deleted Dam1 protein. A single Dam1 complex is shown by the dotted line. Scale bars, 25 nm. Reprinted from [73].
Similar articles
- Mechanism of Ska Recruitment by Ndc80 Complexes to Kinetochores.
Janczyk PŁ, Skorupka KA, Tooley JG, Matson DR, Kestner CA, West T, Pornillos O, Stukenberg PT. Janczyk PŁ, et al. Dev Cell. 2017 May 22;41(4):438-449.e4. doi: 10.1016/j.devcel.2017.04.020. Dev Cell. 2017. PMID: 28535377 Free PMC article. - Mechanisms of force generation by end-on kinetochore-microtubule attachments.
Joglekar AP, Bloom KS, Salmon ED. Joglekar AP, et al. Curr Opin Cell Biol. 2010 Feb;22(1):57-67. doi: 10.1016/j.ceb.2009.12.010. Epub 2010 Jan 12. Curr Opin Cell Biol. 2010. PMID: 20061128 Free PMC article. Review. - Rings, bracelets, sleeves, and chevrons: new structures of kinetochore proteins.
Davis TN, Wordeman L. Davis TN, et al. Trends Cell Biol. 2007 Aug;17(8):377-82. doi: 10.1016/j.tcb.2007.08.001. Epub 2007 Sep 4. Trends Cell Biol. 2007. PMID: 17766118 Free PMC article. Review. - How the SAC gets the axe: Integrating kinetochore microtubule attachments with spindle assembly checkpoint signaling.
Agarwal S, Varma D. Agarwal S, et al. Bioarchitecture. 2015;5(1-2):1-12. doi: 10.1080/19490992.2015.1090669. Epub 2015 Oct 2. Bioarchitecture. 2015. PMID: 26430805 Free PMC article. Review. - Structure of the DASH/Dam1 complex shows its role at the yeast kinetochore-microtubule interface.
Jenni S, Harrison SC. Jenni S, et al. Science. 2018 May 4;360(6388):552-558. doi: 10.1126/science.aar6436. Science. 2018. PMID: 29724956 Free PMC article.
Cited by
- CAMP (C13orf8, ZNF828) is a novel regulator of kinetochore-microtubule attachment.
Itoh G, Kanno S, Uchida KS, Chiba S, Sugino S, Watanabe K, Mizuno K, Yasui A, Hirota T, Tanaka K. Itoh G, et al. EMBO J. 2011 Jan 5;30(1):130-44. doi: 10.1038/emboj.2010.276. Epub 2010 Nov 9. EMBO J. 2011. PMID: 21063390 Free PMC article. - Kinetochore-microtubule coupling mechanisms mediated by the Ska1 complex and Cdt1.
Rahi A, Chakraborty M, Vosberg K, Varma D. Rahi A, et al. Essays Biochem. 2020 Sep 4;64(2):337-347. doi: 10.1042/EBC20190075. Essays Biochem. 2020. PMID: 32844209 Free PMC article. Review. - Self-organization of kinetochore-fibers in human mitotic spindles.
Conway W, Kiewisz R, Fabig G, Kelleher CP, Wu HY, Anjur-Dietrich M, Müller-Reichert T, Needleman DJ. Conway W, et al. Elife. 2022 Jul 25;11:e75458. doi: 10.7554/eLife.75458. Elife. 2022. PMID: 35876665 Free PMC article. - Kinetochore-microtubule interactions: steps towards bi-orientation.
Tanaka TU. Tanaka TU. EMBO J. 2010 Dec 15;29(24):4070-82. doi: 10.1038/emboj.2010.294. Epub 2010 Nov 23. EMBO J. 2010. PMID: 21102558 Free PMC article. Review. - The life and miracles of kinetochores.
Santaguida S, Musacchio A. Santaguida S, et al. EMBO J. 2009 Sep 2;28(17):2511-31. doi: 10.1038/emboj.2009.173. Epub 2009 Jul 23. EMBO J. 2009. PMID: 19629042 Free PMC article. Review.
References
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. - PubMed
- Maiato H, Deluca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci. 2004;117:5461–5477. - PubMed
- Cheeseman I, Desai A. Molecular Architecture of the Kinetochore-Microtubule Interface. Nat Rev Mol Cell Biol. in press. - PubMed
- Tanaka TU, Stark MJ, Tanaka K. Kinetochore capture and bi-orientation on the mitotic spindle. Nat Rev Mol Cell Biol. 2005;6:929–942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM 074215/GM/NIGMS NIH HHS/United States
- R01 GM074215/GM/NIGMS NIH HHS/United States
- CRUK_/Cancer Research UK/United Kingdom
- R01 GM074215-03/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources