Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats - PubMed (original) (raw)
Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats
Norbert Fülöp et al. Biogerontology. 2008 Jun.
Abstract
Changes in the levels of O-linked N-acetyl-glucosamine (O-GlcNAc) on nucleocytoplasmic protein have been associated with a number of age-related diseases such as Alzheimer's and diabetes; however, there is relatively little information regarding the impact of age on tissue O-GlcNAc levels. Therefore, the goal of this study was to determine whether senescence was associated with alterations in O-GlcNAc in heart, aorta, brain and skeletal muscle and if so whether there were also changes in the expression of enzymes critical in regulating O-GlcNAc levels, namely, O-GlcNAc transferase (OGT), O-GlcNAcase and glutamine:fructose-6-phosphate amidotransferase (GFAT). Tissues were harvested from 5- and 24-month old Brown-Norway rats; UDP-GlcNAc, a precursor of O-GlcNAc was assessed by HPLC, O-GlcNAc and OGT levels were assessed by immunoblot analysis and GFAT1/2, OGT, O-GlcNAcase mRNA levels were determined by RT-PCR. In the 24-month old animals serum insulin and triglyceride levels were significantly increased compared to the 5-month old group; however, glucose levels were unchanged. Protein O-GlcNAc levels were significantly increased with age (30-107%) in all tissues examined; however, paradoxically the expression of OGT, which catalyzes O-GlcNAc formation, was decreased by approximately 30% in the heart, aorta and brain. In the heart increased O-GlcNAc was associated with increased UDP-GlcNAc levels and elevated GFAT mRNA while in other tissues we found no difference in UDP-GlcNAc or GFAT mRNA levels. These results demonstrate that senescence is associated with increased O-GlcNAc levels in multiple tissues and support the notion that dysregulation of pathways leading to O-GlcNAc formation may play an important role in the development of age-related diseases.
Figures
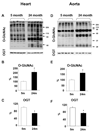
Figure 1
Anti O-GlcNAc and anti OGT Western blot results from hearts (A,B,C) and aortae (D,E,F) of 5- and 24-month old Brown-Norway rats. (A,D), representative Western blots; (B,E), mean area densities of anti O-GlcNAc blots; and (C,F), mean area densities of anti OGT blots. * p<0.05 vs. control.
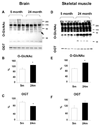
Figure 2
Anti O-GlcNAc and anti OGT Western blot results from brains (A,B,C) and skeletal muscles (D,E,F) of 5- and 24-month old Brown-Norway rats. (A,D), representative Western blots; (B,E), mean area densities of anti O-GlcNAc blots; and (C,F), mean area densities of anti OGT blots. * p<0.05 vs. control.
Figure 3
GFAT1 mRNA levels, GFAT2 mRNA levels as % of 5-month group and UDP-GlcNAc levels in A) heart; B); brain and C) skeletal muscle isolated from 5 and 24 month old Brown-Norway rats, * p<0.05 vs. 5 month old.
Similar articles
- Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart.
Fülöp N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Fülöp N, et al. Am J Physiol Cell Physiol. 2007 Apr;292(4):C1370-8. doi: 10.1152/ajpcell.00422.2006. Epub 2006 Nov 29. Am J Physiol Cell Physiol. 2007. PMID: 17135297 - UDP-N-acetylglucosamine transferase and glutamine: fructose 6-phosphate amidotransferase activities in insulin-sensitive tissues.
Yki-Järvinen H, Vogt C, Iozzo P, Pipek R, Daniels MC, Virkamäki A, Mäkimattila S, Mandarino L, DeFronzo RA, McClain D, Gottschalk WK. Yki-Järvinen H, et al. Diabetologia. 1997 Jan;40(1):76-81. doi: 10.1007/s001250050645. Diabetologia. 1997. PMID: 9028721 - Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure.
Lunde IG, Aronsen JM, Kvaløy H, Qvigstad E, Sjaastad I, Tønnessen T, Christensen G, Grønning-Wang LM, Carlson CR. Lunde IG, et al. Physiol Genomics. 2012 Feb 1;44(2):162-72. doi: 10.1152/physiolgenomics.00016.2011. Epub 2011 Nov 29. Physiol Genomics. 2012. PMID: 22128088 - Reprint of: Role of O-linked N-acetylglucosamine (O-GlcNAc) modification of proteins in diabetic cardiovascular complications.
Chatham JC, Young ME, Zhang J. Chatham JC, et al. Curr Opin Pharmacol. 2020 Oct;54:209-220. doi: 10.1016/j.coph.2020.11.005. Epub 2020 Dec 2. Curr Opin Pharmacol. 2020. PMID: 33278716 Review. - Role of O-linked N-acetylglucosamine (O-GlcNAc) modification of proteins in diabetic cardiovascular complications.
Chatham JC, Young ME, Zhang J. Chatham JC, et al. Curr Opin Pharmacol. 2021 Apr;57:1-12. doi: 10.1016/j.coph.2020.08.005. Epub 2020 Sep 13. Curr Opin Pharmacol. 2021. PMID: 32937226 Free PMC article. Review.
Cited by
- Protein _O_-GlcNAcylation in Cardiac Pathologies: Past, Present, Future.
Ferron M, Denis M, Persello A, Rathagirishnan R, Lauzier B. Ferron M, et al. Front Endocrinol (Lausanne). 2019 Jan 15;9:819. doi: 10.3389/fendo.2018.00819. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30697194 Free PMC article. Review. - Estrogen and mechanisms of vascular protection.
Xing D, Nozell S, Chen YF, Hage F, Oparil S. Xing D, et al. Arterioscler Thromb Vasc Biol. 2009 Mar;29(3):289-95. doi: 10.1161/ATVBAHA.108.182279. Epub 2009 Feb 16. Arterioscler Thromb Vasc Biol. 2009. PMID: 19221203 Free PMC article. Review. - Molecular Mechanisms of Autophagy Decline during Aging.
Lim SHY, Hansen M, Kumsta C. Lim SHY, et al. Cells. 2024 Aug 16;13(16):1364. doi: 10.3390/cells13161364. Cells. 2024. PMID: 39195254 Free PMC article. Review. - Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent.
Zou L, Zhu-Mauldin X, Marchase RB, Paterson AJ, Liu J, Yang Q, Chatham JC. Zou L, et al. J Biol Chem. 2012 Oct 5;287(41):34419-31. doi: 10.1074/jbc.M112.393207. Epub 2012 Aug 20. J Biol Chem. 2012. PMID: 22908225 Free PMC article. - STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology.
Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. Collins HE, et al. Am J Physiol Heart Circ Physiol. 2013 Aug 15;305(4):H446-58. doi: 10.1152/ajpheart.00104.2013. Epub 2013 Jun 21. Am J Physiol Heart Circ Physiol. 2013. PMID: 23792674 Free PMC article. Review.
References
- Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2006 - PubMed
- Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–28744. - PubMed
- Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. - PubMed
- Burattini R, Di Nardo F, Boemi M, Fumelli P. Deterioration of insulin sensitivity and glucose effectiveness with age and hypertension. Am J Hypertens. 2006;19:98–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-076175/HL/NHLBI NIH HHS/United States
- R01 HL067464/HL/NHLBI NIH HHS/United States
- R01 HL079364-04/HL/NHLBI NIH HHS/United States
- HL-077100/HL/NHLBI NIH HHS/United States
- R01 HL076175/HL/NHLBI NIH HHS/United States
- R01 HL079364/HL/NHLBI NIH HHS/United States
- P50 HL077100-040003/HL/NHLBI NIH HHS/United States
- R01 HL067464-08/HL/NHLBI NIH HHS/United States
- P50 HL077100/HL/NHLBI NIH HHS/United States
- HL-67464/HL/NHLBI NIH HHS/United States
- HL079364/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous