Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs - PubMed (original) (raw)
Comparative Study
Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs
Kevin Kwong et al. J Physiol. 2008.
Abstract
Lung vagal sensory fibres are broadly categorized as C fibres (nociceptors) and A fibres (non-nociceptive; rapidly and slowly adapting low-threshold stretch receptors). These afferent fibre types differ in degree of myelination, conduction velocity, neuropeptide content, sensitivity to chemical and mechanical stimuli, as well as evoked reflex responses. Recent studies in nociceptive fibres of the somatosensory system indicated that the tetrodotoxin-resistant (TTX-R) voltage-gated sodium channels (VGSC) are preferentially expressed in the nociceptive fibres of the somatosensory system (dorsal root ganglia). Whereas TTX-R sodium currents have been documented in lung vagal sensory nerves fibres, a rigorous comparison of their expression in nociceptive versus non-nociceptive vagal sensory neurons has not been carried out. Using multiple approaches including patch clamp electrophysiology, immunohistochemistry, and single-cell gene expression analysis in the guinea pig, we obtained data supporting the hypothesis that the TTX-R sodium currents are similarly distributed between nodose ganglion A-fibres and C-fibres innervating the lung. Moreover, mRNA and immunoreactivity for the TTX-R VGSC molecules Na(V)1.8 and Na(V)1.9 were present in nearly all neurons. We conclude that contrary to findings in the somatosensory neurons, TTX-R VGSCs are not preferentially expressed in the nociceptive C-fibre population innervating the lungs.
Figures
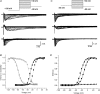
Figure 1. Voltage-gated sodium currents in lung-specific nodose ganglion neurons
A, pharmacological method for isolating TTX-R _I_Na. Families of currents evoked by 30 ms voltage steps from −90 mV to 30 mV in 5 mV increments from a holding potential of −120 mV. Inset: voltage protocol. Total _I_Na was recorded in a reduced sodium solution (upper traces). Only 20 ms is depicted for clarity. TTX-R _I_Na was recorded in the presence of 0.3 μ
m
TTX (middle traces). Digital subtraction of the TTX-R _I_Na from the total _I_Na revealed the TTX-S component (lower traces). B, activation and steady-state inactivation curves for TTX-S _I_Na (n = 33 and 29, respectively) and TTX-R _I_Na (n = 39 and 31, respectively). Circles represent TTX-S _I_Na. Squares represent TTX-R _I_Na. Filled symbols, activation. Open symbols, steady-state inactivation. Continuous and dotted lines represent Boltzmann fits for TTX-S _I_Na and TTX-R _I_Na, respectively. G is conductance. C, prepulse inactivation method for isolating TTX-R _I_Na. In a separate neuron, total _I_Na was recorded in a reduced sodium solution using the identical voltage protocol in A (upper traces). Middle traces: TTX-R-like _I_Na was generated using a prepulse inactivation step to −55 mV for 500 ms followed by a series of 30 ms steps from −90 mV to 30 mV in 5 mV increments (inset). Digital subtraction of the TTX-R _I_Na from the total _I_Na revealed the TTX-S component (lower traces). D, activation curves for TTX-S _I_Na (n = 23) and TTX-R _I_Na (n = 23). •, TTX-S-like _I_Na. ▪, TTX-R-like _I_Na. ▴, recordings in zero-concentration sodium (n = 4). Data are means ±
s.e.m.
Figure 2. TTX-R _I_Na recorded from intact neurons in the nodose ganglion
A, representative traces from a lung-specific non-nociceptive neuron showing a family of TTX-R I_Na evoked using a prepulse inactivation protocol (inset;_ see Fig. 1 legend). B, activation curves fitted using a Boltzmann function (n = 5). G is conductance. C, action potential elicited with a suprathreshold current step in the presence of TTX (1 μ
m
). Inset depicts an expanded time scale. Data are means ±
s.e.m.
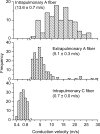
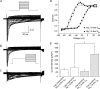
Figure 3. Three distinct phenotypes of nodose fibres innervate airways and lungs of guinea pigs
Histograms of conduction velocities were obtained from recordings in isolated innervated trachea or lung preparation (see methods in Riccio et al. 1996_b_; Undem et al. 2004). Nodose ganglion fibres innervating the intrapulmonary compartment segregate into two populations. Upper panel: the faster conducting group (n = 114) has a conduction velocity centred around 13.6 ± 0.7 m s−1, which is classified as Aβ. In general, this population possesses low-threshold mechanosensitivity and is often attributed as stretch receptors. Note the different frequency scale used. Lower panel: the slower conducting group (n = 149) has a conduction velocity centred around 0.7 ± 0.01 m s−1, which falls in the C fibre range. Hallmarks of this group include sensitivity to a variety of chemicals, including capsaicin, but relative insensitivity to mechanical stimulation. Middle panel: nodose nerves innervating the extrapulmonary airways (larynx, trachea and main bronchi) have an intermediate conduction velocity centred around 5.1 ± 0.3 m s−1, which is classified as Aδ (n = 190). This population is not activated by capsaicin, but is highly sensitive to punctate mechanical stimulation, which evokes cough in intact animals. Histograms in upper, middle, and lower panels were binned at 0.1, 1, and 2 m s−1, respectively. Data are means ±
s.e.m.
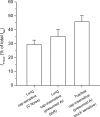
Figure 4. TTX-R _I_Na in three distinct phenotypes of nodose ganglion neurons innervating the lungs and airways
TTX-R _I_Na as a percentage of total _I_Na was evaluated in lung-specific capsaicin-sensitive (n = 9), lung-specific capsaicin-insensitive (n = 14), and trachea-specific capsaicin-insensitive (n = 13) neurons. Cap is capsaicin. Data are means ±
s.e.m.
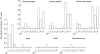
Figure 5. Voltage-gated α subunit mRNA expression in guinea pig tissues
Quantitative real-time RT-PCR was used to determine expression of 9 sodium channel α subunits in a variety of guinea pig tissues. Tissues studied were nodose ganglia (n = 3), jugular ganglia (n = 3), dorsal root ganglia (n = 3), brain tissue (n = 2), heart (n = 3), and skeletal muscle (n = 2). Note that relative amounts of mRNA within a given tissue type are approximate as the relative efficiencies of the RT step for each of the 9 NaVs is unknown. Data are means ±
s.e.m.
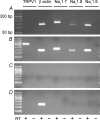
Figure 6. Coexpression of NaV1.7, NaV1.8 and NaV1.9 α subunits in lung-specific nodose neurons
Single-cell RT-PCR was used to evaluate coincident expression of α subunit of NaV1.7, NaV1.8 and NaV1.9 in TRPV1-positive (presumed capsaicin-sensitive) and TRPV1-negative (presumed capsaicin-insensitive) lung-specific nodose ganglion neurons. Examples of TRPV1-negative (A) and TRPV1-positive (B) neurons are depicted. C, no signals were detected in bath controls. D, non-neuronal material surrounding the cells was also evaluated. Message for β actin was routinely detected. PCR products were run on 1.5% agarose gels. Left-most lane represents a 50 bp ladder. Predicted sizes for TRPV1, β-actin, NaV1.7, NaV1.8 and NaV1.9 were 284 bp, 132 bp, 158 bp, 123 bp and 117 bp, respectively.
Figure 7. Coexpression of NaV1.8-like and NaV1.9-like _I_Na in a majority of lung-labelled nodose ganglion neurons
A, TTX-R _I_Na evoked with 100 ms voltage steps from −90 mV to 30 mV in 5 mV increments in the presence of 0.3 μ
m
TTX. Holding potential was −120 mV. Fluoride was the primary anion in the pipette solution in this set of experiments. Inset: voltage protocol. B, a 500 ms prepulse to −40 mV prior to voltage steps protocol in A yielded the NaV1.8-like current. Inset: voltage protocol. C, digital subtraction of the NaV1.8-like current from the total TTX-R _I_Na revealed the NaV1.9-like current. D, activation curves for NaV1.8 (•; n = 17) and NaV1.9 (▪; n = 13). Data were fitted with Boltzmann function (line). E, comparison of NaV1.8 and NaV1.9 current density among capsaicin-sensitive (hatched bars; n = 5) and -insensitive (open bars; n = 13) lung-specific nodose ganglion neurons. *Statistically significant comparison (P < 0.05). Data are means ±
s.e.m.
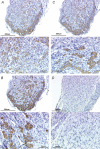
Figure 8. Immunoreactivity for NaV1.7, NaV1.8, and NaV1.9 found in virtually all cells in nodose ganglion
A, representative low magnification (top) and high magnification (bottom) photomicrograph showing NaV1.7-like immunoreactivity using 1 : 1500 primary antibody concentration. Immunoreactivity was visualized using the chromagen DAG/horseradish peroxidase reaction. Mayer's haematoxylin was used to counterstain the tissues. B, low magnification (top) and high magnification (bottom) photomicrographs showing NaV1.8-like immunoreactivity (1 : 3000). C, low magnification (top) and high magnification (bottom) photomicrographs showing NaV1.9-like immunoreactivity (1 : 1500). D, rabbit IgG isotype control (2.5 μg ml−1). Top and bottom photomicrographs are at low and high magnification, respectively. Bars indicate 300 μm and 100 μm for low magnification and high magnification photomicrographs.
Similar articles
- Different role of TTX-sensitive voltage-gated sodium channel (NaV 1) subtypes in action potential initiation and conduction in vagal airway nociceptors.
Kollarik M, Sun H, Herbstsomer RA, Ru F, Kocmalova M, Meeker SN, Undem BJ. Kollarik M, et al. J Physiol. 2018 Apr 15;596(8):1419-1432. doi: 10.1113/JP275698. J Physiol. 2018. PMID: 29435993 Free PMC article. - Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons.
Blair NT, Bean BP. Blair NT, et al. J Neurosci. 2002 Dec 1;22(23):10277-90. doi: 10.1523/JNEUROSCI.22-23-10277.2002. J Neurosci. 2002. PMID: 12451128 Free PMC article. - Analysis of the variation in use-dependent inactivation of high-threshold tetrodotoxin-resistant sodium currents recorded from rat sensory neurons.
Tripathi PK, Trujillo L, Cardenas CA, Cardenas CG, de Armendi AJ, Scroggs RS. Tripathi PK, et al. Neuroscience. 2006 Dec 28;143(4):923-38. doi: 10.1016/j.neuroscience.2006.08.052. Epub 2006 Oct 4. Neuroscience. 2006. PMID: 17027172 - Sensory input to the central nervous system from the lungs and airways: A prominent role for purinergic signalling via P2X2/3 receptors.
Adriaensen D, Brouns I, Timmermans JP. Adriaensen D, et al. Auton Neurosci. 2015 Sep;191:39-47. doi: 10.1016/j.autneu.2015.04.006. Epub 2015 Apr 29. Auton Neurosci. 2015. PMID: 25953244 Review. - Tetrodotoxin (TTX) as a therapeutic agent for pain.
Nieto FR, Cobos EJ, Tejada MÁ, Sánchez-Fernández C, González-Cano R, Cendán CM. Nieto FR, et al. Mar Drugs. 2012 Feb;10(2):281-305. doi: 10.3390/md10020281. Epub 2012 Jan 31. Mar Drugs. 2012. PMID: 22412801 Free PMC article. Review.
Cited by
- The Nav1.9 channel regulates colonic motility in mice.
Copel C, Clerc N, Osorio N, Delmas P, Mazet B. Copel C, et al. Front Neurosci. 2013 Apr 15;7:58. doi: 10.3389/fnins.2013.00058. eCollection 2013. Front Neurosci. 2013. PMID: 23596386 Free PMC article. - Activation of TREK currents by riluzole in three subgroups of cultured mouse nodose ganglion neurons.
Fernández-Fernández D, Cadaveira-Mosquera A, Rueda-Ruzafa L, Herrera-Pérez S, Veale EL, Reboreda A, Mathie A, Lamas JA. Fernández-Fernández D, et al. PLoS One. 2018 Jun 21;13(6):e0199282. doi: 10.1371/journal.pone.0199282. eCollection 2018. PLoS One. 2018. PMID: 29928032 Free PMC article. - Spinal cord injury-mediated changes in electrophysiological properties of rat gastric nodose ganglion neurons.
Blanke EN, Ruiz-Velasco V, Holmes GM. Blanke EN, et al. Exp Neurol. 2022 Feb;348:113927. doi: 10.1016/j.expneurol.2021.113927. Epub 2021 Nov 16. Exp Neurol. 2022. PMID: 34798136 Free PMC article. - Action potential conduction in the mouse and rat vagus nerve is dependent on multiple voltage-gated sodium channels (NaV1s).
Nair SS, Pavelkova N, Murphy CM, Kollarik M, Taylor-Clark TE. Nair SS, et al. J Neurophysiol. 2023 Sep 1;130(3):684-693. doi: 10.1152/jn.00041.2023. Epub 2023 Aug 16. J Neurophysiol. 2023. PMID: 37584077 Free PMC article. - Targeting voltage gated sodium channels NaV1.7, Na V1.8, and Na V1.9 for treatment of pathological cough.
Muroi Y, Undem BJ. Muroi Y, et al. Lung. 2014 Feb;192(1):15-20. doi: 10.1007/s00408-013-9533-x. Epub 2013 Nov 24. Lung. 2014. PMID: 24272479 Free PMC article. Review.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. - PMC - PubMed
- Arbuckle JB, Docherty RJ. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995;185:70–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources