Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing - PubMed (original) (raw)
Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing
Kenneth P K Lee et al. Cell. 2008.
Abstract
Ire1 is an ancient transmembrane sensor of ER stress with dual protein kinase and ribonuclease activities. In response to ER stress, Ire1 catalyzes the splicing of target mRNAs in a spliceosome-independent manner. We have determined the crystal structure of the dual catalytic region of Ire1at 2.4 A resolution, revealing the fusion of a domain, which we term the KEN domain, to the protein kinase domain. Dimerization of the kinase domain composes a large catalytic surface on the KEN domain which carries out ribonuclease function. We further show that signal induced trans-autophosphorylation of the kinase domain permits unfettered binding of nucleotide, which in turn promotes dimerization to compose the ribonuclease active site. Comparison of Ire1 to a topologically disparate ribonuclease reveals the convergent evolution of their catalytic mechanism. These findings provide a basis for understanding the mechanism of action of RNaseL and other pseudokinases, which represent 10% of the human kinome.
Figures
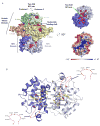
Figure 1. Sequence conservation in the dual-catalytic core of Ire1
Structure based sequence alignment of the protein kinase domain and KEN (
K
inase
E
xtension
N
uclease) domain of Ire1 sequences from S. cerevisiae (yeast), H. sapiens (human), M. musculus (mouse), R. norvegicus (rat), G. gallus (chicken), X. laevis (frog), D. melanogaster (fruitfly), A. mellifera (honey bee), C. elegans (nematode), A. thaliana (mouse-ear cress), and O. sativa (rice); and RNase L sequences from H. sapiens (human), P. pygmaeus (orangutan), M. musculus (mouse), R. norvegicus (rat), and C. familiarus (dog). Residues invariant across the Ire1-RNaseL family of kinase-ribonucleases are highlighted in blue, while residues invariant or highly conserved specifically in Ire1 are colored in green and yellow, respectively. The secondary structure of S. cerevisiae Ire1 is indicated above the alignment using the coloring scheme of Protomer 1 in Figure 2A. A conserved disordered region in the KEN domain termed “Putative RNA specificity determinants” is delineated by a black-box. The kinase domain activation segment of Ire1 is indicated by a set of black arrows. Dashed lines represent unmodeled disordered regions of the crystal structure.
Figure 2. Structure of Ire1
(A) Ribbons representation of the dimeric dual-catalytic region of Ire1 encompassing the protein kinase domain and KEN domain. The kinase N-lobe, C-lobe and the KEN domain are colored green, orange and blue (protomer 1) and light green, yellow and light blue (protomer 2), respectively. ADP and coordinating metal ions are shown within the kinase domain catalytic cleft. Two internal regions within the kinase domain and one internal region within the KEN domain not modeled due to disorder are shown as dashed lines. (B) A continuous hydrophobic core connects the kinase C-lobe and the KEN domain. Residues originating from the C-lobe and KEN domain are colored in pink and green, respectively. Ribbons diagram color scheme corresponds to that of protomer 2 in (A). (C) Detailed stereoview of the KEN domain dimer as shown in (A). Disordered regions corresponding to putative RNA specificity determinants are shown as red dashed lines. (D) and (E) Dimer interface interactions involving conserved residues in the kinase domain (D) and the KEN domain (E), respectively, viewed along the two fold axis of rotational symmetry. For clarity, only residues targeted for mutation in this study are shown in ball and stick representation. Residues colored yellow and white originate from promoter 1 and 2, respectively. Ribbons coloring scheme corresponds to that in (A).
Figure 3. Sequence conservation of Ire1 reveals the ribonuclease active site
(A) Surface representations of the Ire1cyto dimer. Frontal view (left panel) and bottom view (top right panel) are coloured as in Figure 2A with all highly conserved/invariant residues highlighted in red. Bottom right panel displays a bottom view of Ire1cyto coloured according to electrostatic potential. The axis of two-fold non-crystallographic symmetry is indicated in each view. (B) Expanded bottom view of a highly conserved surface on the KEN domain. Invariant or highly conserved side chains are displayed in yellow ball and stick representation. The red dashed lines indicate the position of disordered linkers corresponding to putative determinant of RNA specificity. Residues within the disordered linker are shown in inset.
Figure 4. Kinase domain and KEN domain dimerization surfaces are important for Ire1 ribonuclease function
(A) Autoradiograms of radiolabelled Xbp1mini cleavage products generated by wild type and the indicated single-site dimer interface mutants of Ire1cyto (top) or single-site and double-site charge reversal mutants of Ire1cyto (bottom). The percentage substrate cleaved is indicated below each lane. Note the marked improvement in cleavage efficiency observed when the charge reversal mutations R697D and D723R or D723R and R730D are combined. All cleavage reactions were performed using identical enzyme and substrate concentrations. (B) _Ire1_-null yeast strains transformed with wild type or the indicated dimer interface mutants of full-length FLAG tagged-Ire1 were grown to saturation and 4-μl of 10-fold serial dilutions (starting OD600 = 1.0) were spotted on minimal medium supplemented with essential nutrients (SD) or medium containing 0.25 μg/ml tunicamycin. (Bottom panel) Western analysis of Ire1 expression in yeast. 30-μg of yeast whole-cell extracts, prepared as described previously (Kimata et al., 2004), were separated by SDS-PAGE and then immunoblotted using monoclonal anti-FLAG antibodies (Sigma) or with anti-eIF2α antibodies as a control.
Figure 5. Disruption of the putative ribonuclease active site abrogates ribonuclease function
(A) Autoradiograms of radiolabelled Xbp1mini cleavage products generated by wild type and the indicated single-site mutants of Ire1cyto within the ordered conserved surface of the KEN domain (top) and the disordered conserved linker adjoining helix α3 and α4 (bottom). (B) _Ire1_-null yeast strains transformed with wild type or the indicated KEN domain single-site mutants of full-length FLAG tagged-Ire1 were spotted on minimal medium supplemented with essential nutrients (SD) or medium containing 0.25 μg/ml tunicamycin. (Bottom panels) Western analyses of Ire1 expression in yeast. 30-μg of yeast whole-cell extracts, prepared as described previously (Kimata et al., 2004), were separated by SDS-PAGE and then immunoblotted using monoclonal anti-FLAG antibodies (Sigma).
Figure 6. Ire1 autophosphorylation enables nucleotide binding
Isothermal titration calorimetry profiles obtained by titration of ADP against hyperphosphorylated wildtype Ire1cyto (left), a non-phosphorylated catalytically inactive Ire1cyto(D797A) mutant (middle), and protein buffer control (right). The dissociation constant (Kd) and stoichiometry (N) obtained by fitting to a one-site binding model for wildtype Ire1 are shown in inset.
Figure 7. Convergent evolution of ribonuclease active site
(A) Structural alignment illustrating concordance in the three-dimensional disposition of ribonuclease catalytic residues in Ire1 and tRNA endonuclease. Sidechains of catalytic residues in the KEN domain of Ire1 are colored blue and the conserved disordered linker connecting helices α3 and α4 is demarked as a red dashed line (top). Catalytic residues in the ribonuclease active site of a topologically disparate splicing ribonuclease – tRNA endonuclease (PDB ID = 2GJW) are shown in pink, while the bound RNA substrate analogue is colored in yellow (middle). For clarity, only the scissile phosphate and the flanking nucleotides are shown. RNA-protein interactions are indicated with black dashed lines. Functionally analogous residues in Ire1 (N1057, R1056 and H1061) and tRNA endonuclease (S258, R1056 and H257) align very well, with the exception of Y1043 in Ire1, which is displaced from the functionally equivalent Y246 in tRNA endonuclease by 5Å (bottom). (B) Model of dimerization induced signaling by Ire1 across the ER membrane.
Comment in
- How IRE1 reacts to ER stress.
Ron D, Hubbard SR. Ron D, et al. Cell. 2008 Jan 11;132(1):24-6. doi: 10.1016/j.cell.2007.12.017. Cell. 2008. PMID: 18191217 Review.
Similar articles
- How IRE1 reacts to ER stress.
Ron D, Hubbard SR. Ron D, et al. Cell. 2008 Jan 11;132(1):24-6. doi: 10.1016/j.cell.2007.12.017. Cell. 2008. PMID: 18191217 Review. - On the mechanism of sensing unfolded protein in the endoplasmic reticulum.
Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. Credle JJ, et al. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):18773-84. doi: 10.1073/pnas.0509487102. Epub 2005 Dec 19. Proc Natl Acad Sci U S A. 2005. PMID: 16365312 Free PMC article. - The unfolded protein response signals through high-order assembly of Ire1.
Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. Korennykh AV, et al. Nature. 2009 Feb 5;457(7230):687-93. doi: 10.1038/nature07661. Epub 2008 Dec 14. Nature. 2009. PMID: 19079236 Free PMC article. - Bypassing a kinase activity with an ATP-competitive drug.
Papa FR, Zhang C, Shokat K, Walter P. Papa FR, et al. Science. 2003 Nov 28;302(5650):1533-7. doi: 10.1126/science.1090031. Epub 2003 Oct 16. Science. 2003. PMID: 14564015 - Translation Control of HAC1 by Regulation of Splicing in Saccharomyces cerevisiae.
Xia X. Xia X. Int J Mol Sci. 2019 Jun 12;20(12):2860. doi: 10.3390/ijms20122860. Int J Mol Sci. 2019. PMID: 31212749 Free PMC article. Review.
Cited by
- Structural and Functional Analysis of the Allosteric Inhibition of IRE1α with ATP-Competitive Ligands.
Feldman HC, Tong M, Wang L, Meza-Acevedo R, Gobillot TA, Lebedev I, Gliedt MJ, Hari SB, Mitra AK, Backes BJ, Papa FR, Seeliger MA, Maly DJ. Feldman HC, et al. ACS Chem Biol. 2016 Aug 19;11(8):2195-205. doi: 10.1021/acschembio.5b00940. Epub 2016 Jun 9. ACS Chem Biol. 2016. PMID: 27227314 Free PMC article. - Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing.
Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R, Iwawaki T, Imoto M, Iida S. Ri M, et al. Blood Cancer J. 2012 Jul;2(7):e79. doi: 10.1038/bcj.2012.26. Epub 2012 Jul 20. Blood Cancer J. 2012. PMID: 22852048 Free PMC article. - METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction.
Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, Hotamisligil GS. Yang L, et al. Science. 2015 Jul 31;349(6247):500-6. doi: 10.1126/science.aaa0079. Science. 2015. PMID: 26228140 Free PMC article. - UPR-induced resistance to etoposide is downstream of PERK and independent of changes in topoisomerase IIα levels.
Mann MJ, Pereira ER, Liao N, Hendershot LM. Mann MJ, et al. PLoS One. 2012;7(10):e47931. doi: 10.1371/journal.pone.0047931. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144714 Free PMC article. - Multiple Ways for Stress Sensing and Regulation of the Endoplasmic Reticulum-stress Sensors.
Le QG, Kimata Y. Le QG, et al. Cell Struct Funct. 2021 May 22;46(1):37-49. doi: 10.1247/csf.21015. Epub 2021 Mar 26. Cell Struct Funct. 2021. PMID: 33775971 Free PMC article. Review.
References
- Bernales S, Papa FR, Walter P. Intracellular Signaling by the Unfolded Protein Response. Annu Rev Cell Dev Biol 2006 - PubMed
- Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. - PubMed
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases