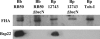Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses - PubMed (original) (raw)
Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses
Neil K Fennelly et al. Infect Immun. 2008 Mar.
Abstract
Certain bacteria use a type III secretion system (TTSS) to deliver effector proteins that interfere with cell function into host cells. While transcription of genes encoding TTSS components has been demonstrated, studies to date have failed to identify TTSS effector proteins in Bordetella pertussis. Here we present the first evidence of a functionally active TTSS in B. pertussis. Three known TTSS effectors, Bsp22, BopN, and BopD, were identified as TTSS substrates in B. pertussis 12743. We found expression of Bsp22 in a significant proportion of clinical isolates but not in common laboratory-adapted strains of B. pertussis. We generated a TTSS mutant of B. pertussis 12743 and showed that it induced significantly lower respiratory tract colonization in mice than the wild-type bacteria. Respiratory infection of mice with the mutant bacteria induced significantly greater innate proinflammatory cytokine production in the lungs soon after challenge, and this correlated with significantly higher antigen-specific interleukin-17, gamma interferon, and immunoglobulin G responses later in infection. Our findings suggest that the TTSS subverts innate and adaptive immune responses during infection of the lungs and may be a functionally important virulence factor for B. pertussis infection of humans.
Figures
FIG. 1.
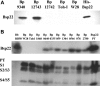
Clinical isolates but not common laboratory-adapted stains of B. pertussis express Bsp22. Protein samples from supernatants of stationary-phase cultures of B. pertussis (Bp) 9340, 12743, 12742, Tohama I (Toh-I), and Wellcome 28 (W28) (A) or B. bronchiseptica (Bb) RB50, B. pertussis W28 and Toh-I, and eight clinical isolates of B. pertussis recovered from respiratory tracts of patients with whooping cough (B), prepared by precipitation with 10% trichloroacetic acid or purified His-Bsp22 or PT (2 μg), were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and probed with Abs specific for Bsp22 or PT (S1 to S5 subunits) by Western blotting.
FIG. 2.
Mutation of bscN in Bordetella spp. abolishes Bsp22 production but does not affect secretion of the virulence factor FHA. Proteins from culture supernatants of WT and Δ_bscN B. pertussis_ (Bp) 12743 and B. bronchiseptica (Bb) RB50 or B. pertussis Tohama I (Toh-I) were examined for the presence of Bsp22 and FHA by Western blotting.
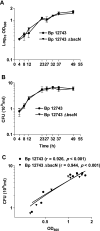
FIG. 3.
Correlation between in vitro growth curves for B. pertussis (Bp) 12743 and Δ_bscN B. pertussis_ 12743. B. pertussis 12743 and Δ_bscN B. pertussis_ 12743 were cultured for 2 days in SS medium, and samples were removed at the indicated time points. (A) OD600 was determined. (B) The number of viable bacteria was determined by performing CFU counts after plating on BG agar. (C) CFU counts were plotted against OD600 for each strain separately, and linear regression analysis was performed. The correlation (r value) and level of significance (P value) are shown.
FIG. 4.
The TTSS of B. pertussis 12743 promotes bacterial adherence to macrophages but does not mediate cytotoxicity. (A) Epithelial cells, J774 macrophages, or bone marrow-derived DC were cultured with WT or Δ_bscN B. pertussis_ (Bp) 12743 or B. bronchiseptica (Bb) RB50 at MOIs of 20, 100, and 500 for 4 h. Cytotoxicity was measured by a lactate dehydrogenase release assay. (B and C) J774 macrophages were cultured with WT or Δ_bscN B. bronchiseptica_ RB50 (B) or B. pertussis 12743 (C) at an MOI of 100:1. Adherence was assessed by assessing CFU counts following 2 h of incubation followed by 1 h of treatment with kanamycin or medium only. *, P < 0.05; **, P < 0.01 (Δ_bscN_ versus WT). Results are representative of three experiments.
FIG. 5.
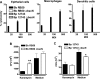
Enhanced inflammatory cytokine and chemokine induction in the lungs of mice infected with Δ_bscN B. pertussis_ (BP) 12743. BALB/c mice were challenged with an aerosol of WT or Δ_bscN B. pertussis_ 12743. Cytokine and chemokine concentrations were determined by ELISA on lung homogenates from mice 3 h and 3, 7, and 14 days (d) after aerosol challenge and in uninfected control mice. Results are means ± standard deviations for four mice per group at each time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Δ_bscN_ versus WT).
FIG. 6.
Enhanced antigen-specific IL-17 and IFN-γ production in mice infected with Δ_bscN B. pertussis_ (BP) 12743. BALB/c mice were challenged with an aerosol of WT or Δ_bscN B. pertussis_ 12743. Spleen cells recovered 14 (A and C) and 21 (B and D) days postchallenge were stimulated with sonicated B. pertussis 12743 (Bp), FHA, PT, or medium (Med) only as a control. Supernatants were removed after 3 days, and IL-17 (A and B) and IFN-γ (C and D) concentrations were determined by ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Δ_bscN_ versus WT).
FIG. 7.
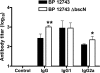
Enhanced B. pertussis_-specific IgG and IgG2a responses in mice infected with Δ_bscN B. pertussis (BP) 12743. BALB/c mice were challenged with an aerosol of WT or Δ_bscN B. pertussis_ 12743. Serum was recovered from infected mice 28 days postchallenge or from uninfected control mice and assayed for anti-B. pertussis IgG, IgG1, and IgG2a by ELISA. Results are means ± standard deviations for four mice per group. *, P < 0.05; **, P < 0.01 (Δ_bscN_ versus WT).
FIG. 8.
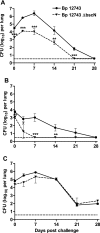
Δ_bscN B. pertussis_ 12743 has reduced ability to colonize lungs of mice. BALB/c mice were challenged with an aerosol of WT or Δ_bscN B. pertussis_ (Bp) 12743, where the challenge inoculum resulted in intermediate (A), low (B), or high (C) initial colonization with the WT B. pertussis. Groups of four mice were sacrificed 3 h and 3, 7, 14, 21, and 28 days later, and CFU counts were performed on lung homogenates. The dashed line represents the limit of detection. **, P < 0.01; ***, P < 0.001 (Δ_bscN_ versus WT). Results are representative of three experiments for panel A and one experiment each for panels B and C.
Similar articles
- Transcriptional profiling of Bordetella pertussis reveals requirement of RNA chaperone Hfq for Type III secretion system functionality.
Bibova I, Hot D, Keidel K, Amman F, Slupek S, Cerny O, Gross R, Vecerek B. Bibova I, et al. RNA Biol. 2015;12(2):175-85. doi: 10.1080/15476286.2015.1017237. RNA Biol. 2015. PMID: 25674816 Free PMC article. - Reciprocal protective immunity against Bordetella pertussis and Bordetella parapertussis in a murine model of respiratory infection.
Watanabe M, Nagai M. Watanabe M, et al. Infect Immun. 2001 Nov;69(11):6981-6. doi: 10.1128/IAI.69.11.6981-6986.2001. Infect Immun. 2001. PMID: 11598073 Free PMC article. - Demonstration of differential virulence gene promoter activation in vivo in Bordetella pertussis using RIVET.
Veal-Carr WL, Stibitz S. Veal-Carr WL, et al. Mol Microbiol. 2005 Feb;55(3):788-98. doi: 10.1111/j.1365-2958.2004.04418.x. Mol Microbiol. 2005. PMID: 15661004 - Pertussis: a matter of immune modulation.
de Gouw D, Diavatopoulos DA, Bootsma HJ, Hermans PW, Mooi FR. de Gouw D, et al. FEMS Microbiol Rev. 2011 May;35(3):441-74. doi: 10.1111/j.1574-6976.2010.00257.x. Epub 2011 Jan 5. FEMS Microbiol Rev. 2011. PMID: 21204863 Review. - The virulence factors of Bordetella pertussis: talented modulators of host immune response.
Fedele G, Bianco M, Ausiello CM. Fedele G, et al. Arch Immunol Ther Exp (Warsz). 2013 Dec;61(6):445-57. doi: 10.1007/s00005-013-0242-1. Epub 2013 Aug 18. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23955529 Review.
Cited by
- Gut bacterial type III secretion systems aggravate colitis in mice and serve as biomarkers of Crohn's disease.
Xu J, Li P, Li Z, Liu S, Guo H, Lesser CF, Ke J, Zhao W, Mou X. Xu J, et al. EBioMedicine. 2024 Sep;107:105296. doi: 10.1016/j.ebiom.2024.105296. Epub 2024 Aug 30. EBioMedicine. 2024. PMID: 39216231 Free PMC article. - Eosinophils as drivers of bacterial immunomodulation and persistence.
Parrish KM, Gestal MC. Parrish KM, et al. Infect Immun. 2024 Sep 10;92(9):e0017524. doi: 10.1128/iai.00175-24. Epub 2024 Jul 15. Infect Immun. 2024. PMID: 39007622 Free PMC article. Review. - T3SS chaperone of the CesT family is required for secretion of the anti-sigma factor BtrA in Bordetella pertussis.
Držmíšek J, Petráčková D, Dienstbier A, Čurnová I, Večerek B. Držmíšek J, et al. Emerg Microbes Infect. 2023 Dec;12(2):2272638. doi: 10.1080/22221751.2023.2272638. Epub 2023 Nov 1. Emerg Microbes Infect. 2023. PMID: 37850324 Free PMC article. - Cyclic di-GMP Regulates the Type III Secretion System and Virulence in Bordetella bronchiseptica.
Gutierrez MP, Wong TY, Damron FH, Fernández J, Sisti F. Gutierrez MP, et al. Infect Immun. 2022 Jun 16;90(6):e0010722. doi: 10.1128/iai.00107-22. Epub 2022 May 25. Infect Immun. 2022. PMID: 35612302 Free PMC article. - Pathogenicity and virulence of Bordetella pertussis and its adaptation to its strictly human host.
Belcher T, Dubois V, Rivera-Millot A, Locht C, Jacob-Dubuisson F. Belcher T, et al. Virulence. 2021 Dec;12(1):2608-2632. doi: 10.1080/21505594.2021.1980987. Virulence. 2021. PMID: 34590541 Free PMC article. Review.
References
- Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80611-620. - PubMed
- Byrne, P., P. McGuirk, S. Todryk, and K. H. Mills. 2004. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 342579-2588. - PubMed
- Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3742-752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources