Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity - PubMed (original) (raw)
Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity
Zhouhua Li et al. J Cell Biol. 2008.
Abstract
Asymmetrical localization of transcripts coupled with localized translation constitutes an important mechanism widely deployed to regulate gene activity in a spatial manner. The conserved transmembrane protein Crumbs (Crb) is an important regulator of epithelial polarity. However, it remains unclear how Crb is targeted to the apical domain. Here, we show that the cytoplasmic dynein complex transports both Crb protein and transcripts to the apical domain of Drosophila melanogaster follicular cells (FCs). The crb 3' untranslated region (UTR) is necessary and sufficient for the apical localization of its transcript and this apical transcript localization is crucial for crb function. In crb mutant FCs, Crb protein derived from transgenes lacking the 3' UTR does not effectively localize to the apical domain and does not effectively restore normal epithelial polarity. We propose that dynein-mediated messenger RNA transport coupled with a localized translation mechanism is involved in localizing Crb to the apical domain to mediate epithelial apicobasal polarity and that this mechanism might be widely used to regulate cellular polarity.
Figures
Figure 1.
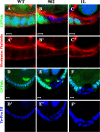
Dynein function is required for FC A/B polarity. GFPnls are shown as green, DNA is shown as blue, and mutant clones are marked by the absence of GFP and apical up unless otherwise stated. (A) wt FCs display regular cuboidal morphology by rhodamine phalloidin staining (red). Dhc64C902 (B) and Gl1L (C) mutant FCs show altered morphology. wt FCs are monolayered (D), whereas Dhc64C902 (E) and Gl1L (F) mutant FCs are multiple layered at the posterior end. Bars, 5 μm.
Figure 2.
Dynein is required for the apical localization of the Crb complex. Rhodamine phalloidin is shown in red and GFPnls is shown in green. Crb (blue) localizes to the apical domain of wt FCs (A) but is lost from the apical domain in the dynein mutant and colchicine-treated FCs (B, C, and M). Arm (blue) localizes to the adherens junctions in wt FCs (D) and this localization is largely normal in dynein mutant and colchicine-treated FCs (E, F, and N). aPKC (blue) localizes to the apical region of wt FCs (G) and is largely unaffected in dynein mutant and colchicine-treated FCs (H, I, and O). Scrib (blue) localizes along the lateral domain of wt FCs (J) and is only slightly apically expanded in the dynein mutant cells (K, L, and P). Bars, 5 μm.
Figure 3.
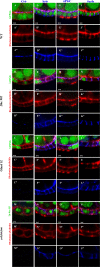
Apical crb transcript localization is required for Crb activity. Crb protein is shown in red, and crb mRNA (green) localizes apically in wt FCs (A) but delocalizes in the dynein mutant (B). Injected crbintra-myc transcripts (red) localize apically in wt (C) and anti-Myc 9E10 antibody–treated (F) but not in P1H4 (anti-Dhc64C) antibody–treated or colchicine-treated blastoderm embryos (D and E). crbintra-myc-wo transcripts (red) fail to localize apically in wt blastoderm embryos (G). lacZ-crb 3′ UTR (H, red) but not lacZ-SV40 3′ UTR transcripts (I, red) localize apically in blastoderm embryos. crbintra-myc transcripts (J) but not crbintra-myc-wo (K) transcripts (green) localize apically in FCs. In J and K, both endogenous and exogenous transcripts are detected. Crbintra-myc protein (green) shows apical localization in wt FCs (L). The majority of Crbintra-myc-wo protein (green) is apically localized in wt FCs (M). In 5% of cells, Crbintra-myc-wo (green) shows cytoplasmic localization (N). Crbintra-myc (O, red) apically localizes in the crb mutant. Crbintra-myc-wo (P, red) is largely cytoplasmic in the crb mutant. In crb mutant FCs, apical localization of Sdt (Q and R, red) is restored by expression of crbintra-myc but not the crbintra-myc-wo transgene (also note the multilayering of mutant cells in R). In Q and R, GFP is in green and mutant cells do not express GFP. Bars, 5 μm.
Figure 4.
Crb and Sdt interact on the apical cortex. Sdt (red) localizes on the apical cortex in wt FCs (A). Sdt localization in newly induced (B) and aged dynein mutant clones (C). Patj/Dlt (red) localizes on the apical domain in wt FCs (D) and gradually localizes into the cytoplasm in the dynein mutant (E, a mutant clone shortly after induction; F, an aged mutant clone). Sdt gradually delocalizes in crb mutant cells (G, early clone; H and I, aged clone). Sdt (red) largely colocalizes with Crbintra-myc (green) on the apical domain in wt FCs (J). Both Sdt and Crbintra-myc become cytoplasmic and do not colocalize in colchicine-treated FCs, which mimic dynein mutant FCs (K). Equal protein loadings of total lysates from wt (left lane) and colchicine-treated ovary (right lane) were probed with an anti-Crb antibody (L); loading control was probed with an anti–α-tubulin antibody (L'). Crbintra-myc and Sdt only form a complex when both localize on the apical cortex (M). Western blot is probed with Sdt. (lane 1) wt (crbintra-myc expressed in wt background) input (10%). (lane 2) Anti-Myc immunoprecipitation from wt sample. (lane 3) Anti-Flag immunoprecipitation (negative control) from wt sample. (lane 4) Anti-Myc immunoprecipitation from a colchicine-treated sample. (lane 5) Anti-Flag immunoprecipitation (control) from a colchicine-treated sample. Bars, 5 μm.
Similar articles
- Dynein regulates epithelial polarity and the apical localization of stardust A mRNA.
Horne-Badovinac S, Bilder D. Horne-Badovinac S, et al. PLoS Genet. 2008 Jan;4(1):e8. doi: 10.1371/journal.pgen.0040008. PLoS Genet. 2008. PMID: 18208331 Free PMC article. - Diamond controls epithelial polarity through the dynactin-dynein complex.
Zhao H, Shi L, Li Z, Kong R, Jia L, Lu S, Wang JH, Dong MQ, Guo X, Li Z. Zhao H, et al. Traffic. 2023 Dec;24(12):552-563. doi: 10.1111/tra.12917. Epub 2023 Aug 29. Traffic. 2023. PMID: 37642208 - The cytoskeletal motor proteins Dynein and MyoV direct apical transport of Crumbs.
Aguilar-Aragon M, Fletcher G, Thompson BJ. Aguilar-Aragon M, et al. Dev Biol. 2020 Mar 15;459(2):126-137. doi: 10.1016/j.ydbio.2019.12.009. Epub 2019 Dec 24. Dev Biol. 2020. PMID: 31881198 Free PMC article. - Role of the Crumbs complex in the regulation of junction formation in Drosophila and mammalian epithelial cells.
Médina E, Lemmers C, Lane-Guermonprez L, Le Bivic A. Médina E, et al. Biol Cell. 2002 Oct;94(6):305-13. doi: 10.1016/s0248-4900(02)00004-7. Biol Cell. 2002. PMID: 12500938 Review. - Establishing and maintaining cell polarity with mRNA localization in Drosophila.
Barr J, Yakovlev KV, Shidlovskii Y, Schedl P. Barr J, et al. Bioessays. 2016 Mar;38(3):244-53. doi: 10.1002/bies.201500088. Epub 2016 Jan 15. Bioessays. 2016. PMID: 26773560 Free PMC article. Review.
Cited by
- Widely conserved signaling pathways in the establishment of cell polarity.
McCaffrey LM, Macara IG. McCaffrey LM, et al. Cold Spring Harb Perspect Biol. 2009 Aug;1(2):a001370. doi: 10.1101/cshperspect.a001370. Cold Spring Harb Perspect Biol. 2009. PMID: 20066082 Free PMC article. Review. - Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions.
Godde NJ, Galea RC, Elsum IA, Humbert PO. Godde NJ, et al. J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):149-68. doi: 10.1007/s10911-010-9180-2. Epub 2010 May 12. J Mammary Gland Biol Neoplasia. 2010. PMID: 20461450 Review. - Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia.
Khanal I, Elbediwy A, Diaz de la Loza Mdel C, Fletcher GC, Thompson BJ. Khanal I, et al. J Cell Sci. 2016 Jul 1;129(13):2651-9. doi: 10.1242/jcs.189076. Epub 2016 May 26. J Cell Sci. 2016. PMID: 27231092 Free PMC article. - Par complex in cancer: a regulator of normal cell polarity joins the dark side.
Aranda V, Nolan ME, Muthuswamy SK. Aranda V, et al. Oncogene. 2008 Nov 24;27(55):6878-87. doi: 10.1038/onc.2008.340. Oncogene. 2008. PMID: 19029931 Free PMC article. Review. - Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity.
Nelson WJ. Nelson WJ. Cold Spring Harb Perspect Biol. 2009 Jul;1(1):a000513. doi: 10.1101/cshperspect.a000513. Cold Spring Harb Perspect Biol. 2009. PMID: 20066074 Free PMC article. Review.
References
- Bachmann, A., M. Schneider, E. Theilenberg, F. Grawe, and E. Knust. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. - PubMed
- Bashirullah, A., R.L. Cooperstock, and H.D. Lipshitz. 1998. RNA localization in development. Annu. Rev. Biochem. 67:335–394. - PubMed
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. - PubMed
- Bilder, D., M. Schober, and N. Perrimon. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58. - PubMed
- Brittis, P.A., Q. Lu, and J.G. Flanagan. 2002. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 110:223–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases