Membrane association and functional regulation of Sec3 by phospholipids and Cdc42 - PubMed (original) (raw)
Membrane association and functional regulation of Sec3 by phospholipids and Cdc42
Xiaoyu Zhang et al. J Cell Biol. 2008.
Abstract
The exocyst is an octameric protein complex implicated in tethering post-Golgi secretory vesicles at the plasma membrane in preparation for fusion. However, it is not clear how the exocyst is targeted to and physically associates with specific domains of the plasma membrane and how its functions are regulated at those regions. We demonstrate that the N terminus of the exocyst component Sec3 directly interacts with phosphatidylinositol 4,5-bisphosphate. In addition, we have identified key residues in Sec3 that are critical for its binding to the guanosine triphosphate-bound form of Cdc42. Genetic analyses indicate that the dual interactions of Sec3 with phospholipids and Cdc42 control its function in yeast cells. Disrupting these interactions not only blocks exocytosis and affects exocyst polarization but also leads to defects in cell morphogenesis. We propose that the interactions of Sec3 with phospholipids and Cdc42 play important roles in exocytosis and polarized cell growth.
Figures
Figure 1.
The gic2-sec3 chimera is able to rescue synthetic lethality between sec3ΔN and exo70-38. (A) sec3ΔN is synthetic lethal with exo70-38. sec3ΔN and exo70-38 were expressed under SEC3 and EXO70 promoters in CEN plasmids. The sec3ΔN exo70-38 double mutant supplemented with a CEN, URA3, SEC3 balancer was streaked out on the plates with (right) or without (left) 5-FOA and incubated for 5 d at 25°C. The sec3ΔN and exo70-38 single mutants were used as controls. exo70-38 sec3ΔN could not survive when losing the SEC3 balancer on the 5-FOA plate (right). (B) Diagram of Sec3 and gic2-sec3 chimera in which the N terminus of Sec3 (aa 1–307) was replaced with the N terminus of Gic2 (aa 1–155; gray). The interaction with Cdc42 is indicated by the arrows. (C) The chimera gic2-sec3 is able to rescue the synthetic lethality between sec3ΔN and exo70-38. gic2-sec3 was expressed under the endogenous SEC3 promoter in a CEN plasmid supplemented with a CEN, URA, SEC3 balancer. Although sec3ΔN and exo70-38 were synthetic lethal, the exo70-38 gic2-sec3 grew well at 25°C in the presence of 5-FOA. (D) The CRIB domain and polybasic region at the N terminus of Gic2 are essential for the functional replacement of wild-type SEC3 with gic2-sec3 chimera in yeast. Chimeras, including gic2-sec3 and _gic2 (CRIB mutation)_-sec3, in which the key residues of the CRIB domain are replaced by alanine (I134A, S135A, and P137A), and gic2 (CRIBΔ)-sec3, gic2 (CRIBΔ & polybasicΔ)-sec3, and gic2-sec3 (K109A, K110A, K119A, K120A, and K121A), in which the polybasic region of Gic2 was mutated, were expressed under the SEC3 promoter in CEN plasmids and tested for their synthetic lethality with exo70-38. All chimeras except gic2-sec3 are synthetic lethal with exo70-38.
Figure 2.
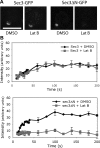
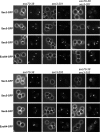
Localization of Sec3 in the tropomyosin mutant and cells treated with latrunculin. (A) The affinity-purified anti-Sec3 antibody recognizes Sec3 in wild-type (SEC3) but not sec3 deletion (sec3Δ) cells by Western blotting (left; molecular masses indicated to the left) and immunofluorescence microscopy (right). (B) Sec3 remains polarized in tpm1-2 tpm2Δ mutant cells. The TPM1 tpm2Δ (left) and tpm1-2 tpm2Δ (right) cells were grown at 25°C and then shifted to 34.5°C for 10 and 60 min before immunostaining with anti-Sec3 or anti-Sec4 antibodies. Both Sec4 and Sec3 were polarized in TPM1 tpm2Δ cells (left). Sec4 was completely depolarized in the tpm1-2 tpm2Δ cells after the temperature shift, whereas Sec3 was mostly polarized, albeit less concentrated than the control cells. Sec3 in these mutants monitored by GFP tagging (Sec3-GFP) was also polarized. (C) Yeast cells were arrested at G0 phase and then released into fresh medium at 25°C for 90 min in the presence of 100 μM Lat B (left) or DMSO (right). Cells were fixed and then processed for immunostaining with the anti-Sec3 antibody. In cells treated with Lat B, Sec3 still formed a patch in the presumptive budding site. Actin cables were disrupted by this treatment. As controls, both Sec3 and actin were polarized in cells treated with DMSO (right). Bars, 5 μm.
Figure 3.
The actin-independent localization of Sec3 is conferred by its N terminus. (A) The targeting of sec3ΔN to the bud tip is dependent on actin. Yeast cells expressing Sec3-GFP or sec3ΔN-GFP under the SEC3 promoter as the sole copy of Sec3 were arrested at G0. Cells were then released into fresh medium at 25°C for 90 min in the presence of 100 μM Lat B or DMSO. Cells were then fixed for fluorescence microscopy. In cells treated with Lat B (left), the full-length Sec3 formed a patch in the presumptive budding site, whereas sec3ΔN-GFP was dispersed throughout the cells (left). As controls, both Sec3-GFP and sec3ΔN-GFP were well polarized in cells treated with DMSO (right). (B) The targeting of gic2-sec3 to the bud tip is independent of actin. gic2-sec3–GFP was expressed under the SEC3 promoter in sec3Δ background. Cells were arrested at G0 phase and released into fresh medium in the presence of 100 μM Lat B. gic2-sec3–GFP was polarized at the presumptive buds when actin was disrupted. Bars, 5 μm.
Figure 4.
FRAP of sec3ΔN-GFP at the bud relies on actin cables. (A) Recovery of Sec3-GFP or sec3ΔN-GFP fluorescence in cells treated with DMSO or 100 μM Lat B. Bar, 5 μm. (B) Fluorescence recovery graphs of cells expressing either Sec3-GFP (top) or sec3ΔN-GFP (bottom) treated with DMSO or Lat B. Each data point represents the mean ± SEM (n = 3; P < 0.05 for every time point after the initial two for sec3ΔN-GFP ± Lat B).
Figure 5.
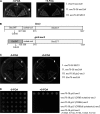
The N terminus of Sec3 directly binds to phospholipids. (A) 0.3 μm GST-Sec3N (aa 1–320) purified from bacteria was incubated with liposomes containing 100% PC, 5% PIP2, 20% PS, 60% PS, or a combination of 5% PIP2 and 20% PS. After ultracentrifugation, proteins in supernatant (S) and pellet (P) were subjected to SDS-PAGE and visualized by SYPRO red staining (top). Sec3N bound to vesicles containing 5% PIP2 and more strongly to vesicles containing both 5% PIP2 and 20% PS. GST did not bind to LUVs with any lipid compositions (bottom). (B) 0.3 μM Sec3N was incubated with increasing concentrations of LUVs composed of 5% PIP2, 20% PS, or 5% PIP2 + 20% PS for the binding reaction. The percentage of lipid-bound Sec3N was plotted with increasing LUV concentration with a single rectangular hyperbola equation (B = BmaxX/[Kd + X]) using SigmaPlot. Each point is the mean of three measurements. Error bars represent SD. (C) Changing the polybasic region to alanine impaired Sec3N binding to phospholipids. Wild-type and mutant Sec3N (sec3N-mt) fusion proteins were incubated with 30 μM of phospholipids containing 2% PIP2 and 20% PS. Proteins in supernatant and pellet were analyzed by SDS-PAGE.
Figure 6.
The Cdc42 binding domain and the polybasic region of Sec3 are important for Sec3 function. (A) Diagram of the Sec3 sequence containing the potential Cdc42 binding site and the polybasic region. Residues before R137 may be involved in lipid binding and residues after I140 may be involved in Cdc42 binding. (B) The sec3-201 mutant was not able to bind to Cdc42 in vitro. GST fusion proteins containing the N terminus (aa 1–320) of Sec3, sec3-201 (I140A, L141A, S142A, and P145A), sec3-202 (K134A, K135A, K136A, and R137A), and sec3-203 (K134E, K135E, K136E, and R137E) were purified and conjugated to glutathione sepharose. Cdc42 was expressed as a Hisx6 fusion and purified from bacteria. The in vitro binding assay was performed using GST-Sec3N and Hisx6-Cdc42 in the presence of GTPγS. The Hisx6-Cdc42 fusion protein bound to the GST-Sec3N sepharose was detected by Western blotting with anti-Hisx6 antibody (top). Equal amounts of wild-type and mutant Sec3 fusion proteins were used in the binding assay (bottom; Ponceau S staining). Cdc42 bound to GST-Sec3N and GST–sec3-202N but not to GST–sec3-201N. GST–sec3-203N has reduced binding to Cdc42. (C) Mutations at the Cdc42 binding domain do not impair Sec3 binding to phospholipids. GST fusion proteins of wild-type Sec3N and sec3-201N mutant were incubated with 30 μM LUV containing 2% PIP2 and 20% PS. Proteins in supernatant and pellet were analyzed by SDS-PAGE. + and −, with or without phospholipids in the protein-lipid binding assays, respectively. (D) Synthetic growth defects of sec3 mutants with exo70-38. sec3-201 has mutations within the Cdc42 binding domain. sec3-202 and sec3-203 have mutations at the polybasic region (K/R→A or K/R→E, respectively). exo70-38, exo70-38 sec3-201, exo70-38 sec3-202, and exo70-38 sec3-203 were serially diluted and spotted onto SC medium plates. Cells were incubated at 25 or 32°C for 5 d. All sec3 mutants showed clear synthetic growth defects with exo70-38 at 32°C, whereas sec3-201 had the greatest defects and was unable to survive with exo70-38 over 32°C. (E) sec3 mutants with combined mutations at both the Cdc42 binding domain and polybasic region are synthetic lethal with exo70-38. Various sec3 mutants expressed under the SEC3 promoter in CEN plasmids were introduced into exo70-38 supplemented with a CEN, URA3, SEC3 balancer. sec3-204 has combined mutations of sec3-201 and sec3-202. sec3-205 combines the mutations of sec3-201 and sec3-203. The cells were serially diluted onto SC plates with or without 5-FOA and incubated for 5 d at 25°C. The sec3-204 and sec3-205 mutants were synthetic lethal with exo70-38 at 25°C.
Figure 7.
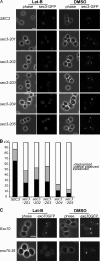
Mutations in the Cdc42 binding region or the polybasic region of Sec3 affect its targeting to the bud tip in the presence of Lat B. (A) Yeast cells expressing SEC3 or various sec3 mutants under the SEC3 promoter as the only copy of Sec3 were GFP tagged and arrested at G0 phase. Cells were then released into fresh medium with 100 μM Lat B (left) or DMSO (right) at 25°C for 90 min. Cells were fixed for fluorescence microscopy. Although the wild-type Sec3 formed a clear patch in the presumptive buds when treated with Lat B, the mutant sec3 proteins were dispersed to different extents. (B) Quantification of the percentages of Sec3-GFP cells that were polarized, partially polarized, or depolarized after treatment with Lat B (n = 300). Partially polarized was scored when the GFP signals were localized at one end of the cell but appeared as multiple patches. (C) Polarization of exo70-38 protein depends on actin. Cells expressing Exo70-GFP or exo70-38–GFP were arrested at G0 phase. These cells were released into fresh medium at 25°C for 90 min in the presence of 100 μM Lat B or DMSO and fixed for fluorescence microscopy. In cells treated with Lat B (left), the wild-type Exo70 still formed a patch in the presumptive budding site. exo70-38–GFP was, however, dispersed throughout the cells. As controls, both Exo70-GFP and exo70-38–GFP were well polarized in cells treated with DMSO. Bars, 5 μm.
Figure 8.
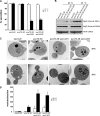
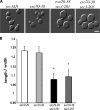
The exo70-38 sec3 double mutants display severe secretion defect. (A) The exo70-38 sec3 double mutants are defective in invertase secretion. The exo70-38 sec3-201 and exo70-38 sec3-203 mutants were tested for the secretion of the invertase after being shifted to the restrictive temperature of 35°C for 2 h. sec3ΔN and exo70-38 mutant strains were used as controls in the assay (n = 3). The percentage of external invertase (secreted) versus total invertase was measured. (B) The exo70-38 sec3 double mutants display aggravated defects in Bgl2 secretion. Western blot analysis of the internal and external pools of Bgl2 in sec3ΔN, exo70-38, exo70-38 sec3-201, and exo70-38 sec3-203 cells. Cells were either grown at 25°C or shifted to 35°C for 2 h. Alcohol dehydrogenase (ADH) was used as a control to show that equal amounts of proteins were loaded. (C) exo70-38 sec3 double mutants accumulate a large amount of secretory vesicles at the restrictive temperature. The sec3ΔN, exo70-38, exo70-38 sec3-201, and exo70-38 sec3-203 cells were grown to early log phase at 25°C (top), and then shifted to 35°C for 2 h and processed for thin-section EM. Bars, 500 nm. (D) Quantification of the number of secretory vesicles per section in the single and double mutant cells (n = 30; P < 0.01). Error bars represent SD.
Figure 9.
Localization of the exocyst components in the exo70-38 sec3 double mutants. The exocyst components in exo70-38, sec3-201, sec3-203, and exo70-38 sec3 double mutant cells were GFP tagged by chromosomal integration. The cells were shifted from 25 to 35°C for 2 h before fluorescence microscopy. GFP-tagged Sec3, 5, and 8 and Exo84 remained polarized to the bud tip in all single mutants but were no longer polarized in double mutants exo70-38 sec3-201 (top) and exo70-38 sec3-203 (bottom) after the temperature shift. Bars, 5 μm.
Figure 10.
Morphological defects of the exo70-38 sec3 double mutants. (A) Morphology of sec3ΔN, exo70-38, exo70-38 sec3-201, and exo70-38 sec3-203. The exo70 sec3 double mutants were significantly larger and rounder than each single mutant, even at 25°C. Bar, 5 μm. (B) Quantification of the mean axial ratios (length/width) of mother cells from each indicated strains. Error bars represent SD among measured samples. Asterisks represent significant difference between mutants and control cells (n = 30; P < 0.05).
Similar articles
- Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth.
Zajac A, Sun X, Zhang J, Guo W. Zajac A, et al. Mol Biol Cell. 2005 Mar;16(3):1500-12. doi: 10.1091/mbc.e04-10-0896. Epub 2005 Jan 12. Mol Biol Cell. 2005. PMID: 15647373 Free PMC article. - Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane.
He B, Xi F, Zhang X, Zhang J, Guo W. He B, et al. EMBO J. 2007 Sep 19;26(18):4053-65. doi: 10.1038/sj.emboj.7601834. Epub 2007 Aug 23. EMBO J. 2007. PMID: 17717527 Free PMC article. - Membrane targeting of the yeast exocyst complex.
Pleskot R, Cwiklik L, Jungwirth P, Žárský V, Potocký M. Pleskot R, et al. Biochim Biophys Acta. 2015 Jul;1848(7):1481-9. doi: 10.1016/j.bbamem.2015.03.026. Epub 2015 Mar 30. Biochim Biophys Acta. 2015. PMID: 25838123 - The exocyst complex in polarized exocytosis.
Hsu SC, TerBush D, Abraham M, Guo W. Hsu SC, et al. Int Rev Cytol. 2004;233:243-65. doi: 10.1016/S0074-7696(04)33006-8. Int Rev Cytol. 2004. PMID: 15037366 Review. - Regulation of yeast polarized exocytosis by phosphoinositide lipids.
Volpiana MW, Nenadic A, Beh CT. Volpiana MW, et al. Cell Mol Life Sci. 2024 Nov 19;81(1):457. doi: 10.1007/s00018-024-05483-x. Cell Mol Life Sci. 2024. PMID: 39560727 Free PMC article. Review.
Cited by
- Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.
Martin TF. Martin TF. Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review. - The exocyst controls lysosome secretion and antigen extraction at the immune synapse of B cells.
Sáez JJ, Diaz J, Ibañez J, Bozo JP, Cabrera Reyes F, Alamo M, Gobert FX, Obino D, Bono MR, Lennon-Duménil AM, Yeaman C, Yuseff MI. Sáez JJ, et al. J Cell Biol. 2019 Jul 1;218(7):2247-2264. doi: 10.1083/jcb.201811131. Epub 2019 Jun 13. J Cell Biol. 2019. PMID: 31197029 Free PMC article. - Integrative structure and function of the yeast exocyst complex.
Ganesan SJ, Feyder MJ, Chemmama IE, Fang F, Rout MP, Chait BT, Shi Y, Munson M, Sali A. Ganesan SJ, et al. Protein Sci. 2020 Jun;29(6):1486-1501. doi: 10.1002/pro.3863. Epub 2020 May 1. Protein Sci. 2020. PMID: 32239688 Free PMC article. - PI(4,5)P₂-binding effector proteins for vesicle exocytosis.
Martin TF. Martin TF. Biochim Biophys Acta. 2015 Jun;1851(6):785-93. doi: 10.1016/j.bbalip.2014.09.017. Epub 2014 Oct 2. Biochim Biophys Acta. 2015. PMID: 25280637 Free PMC article. Review. - Polarized Exocytosis.
Zeng J, Feng S, Wu B, Guo W. Zeng J, et al. Cold Spring Harb Perspect Biol. 2017 Dec 1;9(12):a027870. doi: 10.1101/cshperspect.a027870. Cold Spring Harb Perspect Biol. 2017. PMID: 28246185 Free PMC article. Review.
References
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous