Progressive external ophthalmoplegia and vision and hearing loss in a patient with mutations in POLG2 and OPA1 - PubMed (original) (raw)
Case Reports
doi: 10.1001/archneurol.2007.9.
Susanna Clark, Emanuela Garelli, Guido Davidzon, Steven A Moore, Randy H Kardon, Rachelle J Bienstock, Matthew J Longley, Michelangelo Mancuso, Purificación Gutiérrez Ríos, Michio Hirano, William C Copeland, Salvatore DiMauro
Affiliations
- PMID: 18195150
- PMCID: PMC2364721
- DOI: 10.1001/archneurol.2007.9
Case Reports
Progressive external ophthalmoplegia and vision and hearing loss in a patient with mutations in POLG2 and OPA1
Silvio Ferraris et al. Arch Neurol. 2008 Jan.
Abstract
Objective: To describe the clinical features, muscle pathological characteristics, and molecular studies of a patient with a mutation in the gene encoding the accessory subunit (p55) of polymerase gamma (POLG2) and a mutation in the OPA1 gene.
Design: Clinical examination and morphological, biochemical, and molecular analyses.
Setting: Tertiary care university hospitals and molecular genetics and scientific computing laboratory.
Patient: A 42-year-old man experienced hearing loss, progressive external ophthalmoplegia (PEO), loss of central vision, macrocytic anemia, and hypogonadism. His family history was negative for neurological disease, and his serum lactate level was normal.
Results: A muscle biopsy specimen showed scattered intensely succinate dehydrogenase-positive and cytochrome-c oxidase-negative fibers. Southern blot of muscle mitochondrial DNA showed multiple deletions. The results of screening for mutations in the nuclear genes associated with PEO and multiple mitochondrial DNA deletions, including those in POLG (polymerase gamma gene), ANT1 (gene encoding adenine nucleotide translocator 1), and PEO1, were negative, but sequencing of POLG2 revealed a G1247C mutation in exon 7, resulting in the substitution of a highly conserved glycine with an alanine at codon 416 (G416A). Because biochemical analysis of the mutant protein showed no alteration in chromatographic properties and normal ability to protect the catalytic subunit from N-ethylmaleimide, we also sequenced the OPA1 gene and identified a novel heterozygous mutation (Y582C).
Conclusion: Although we initially focused on the mutation in POLG2, the mutation in OPA1 is more likely to explain the late-onset PEO and multisystem disorder in this patient.
Figures
Figure 1
Histochemical stains for hematoxylin-eosin (A), succinate dehydrogenase (SDH) (B), and cytochrome-c oxidase (COX) (C and D) of serial cross sections of muscle from the patient. In A and C, 2 fibers (labeled 1 and 2) show increased eosinophilia and COX negativity; and in B and D, 2 fibers (labeled 1 and 2) show increased SDH stain and lack of COX activity.
Figure 2
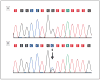
Electropherogram of the POLG2 (gene encoding the accessory subunit [p55] of polymerase γ) sequence showing the heterozygous G1247C transversion in exon 7 (arrow). A, normal sequence; B, patient’s sequence.
Figure 3
Coomassie-stained gel of the purified recombinant G416A-p55 protein.
Figure 4
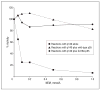
G416A-p55 protects p140 against inactivation by _N_-ethylmaleimide (NEM). DNA polymerase activity was measured as described in the “DNA Polymerase Assays” subsection of the “Methods” section, except that 2-mercaptoethanol was excluded. Reactions contained 75-mmol/L sodium chloride, 8.0 ng (58 fmol) of p140 either alone or in the presence of 6.3 ng (116 fmol) of wild-type p55 or G416A-p55 and the indicated amounts of NEM.
Figure 5
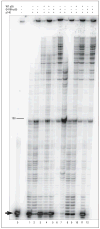
G416A-p55 stimulates processive DNA synthesis of polymerase γ (polγ). Primer extension reactions were performed as described in the “DNA Polymerase Assays” subsection of the “Methods” section. Reactions contained p140 catalytic subunit (lanes 1–6), wild-type (WT) p55 (lanes 3 and 4), G416A-p55 (lanes 5–6), and singly primed M13 DNA. Activity was measured at 0-mmol/L sodium chloride (odd lanes) or 150-mmol/L sodium chloride (even lanes). Lane 0 had no enzyme. Reaction products were resolved by denaturing polyacrylamide gel electrophoresis. The arrow marks the unextended 35-mer primer. The marker 100 indicates the number of nucleotides synthesized past the primer. +; indicates present; −, absent.
Figure 6
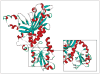
Position of the G416A side chain on the ribbon structure of the human accessory subunit in one monomer within the p55 dimer. The ribbon drawing was generated with Swiss PDB viewer from Protein Data Bank file 2G4C. The inset is an expanded view of the structure around G416 in domain 3.
Similar articles
- Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia.
Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, Nightingale S, Turnbull DM, Copeland WC, Chinnery PF. Longley MJ, et al. Am J Hum Genet. 2006 Jun;78(6):1026-34. doi: 10.1086/504303. Epub 2006 May 4. Am J Hum Genet. 2006. PMID: 16685652 Free PMC article. - Novel Twinkle (PEO1) gene mutations in mendelian progressive external ophthalmoplegia.
Virgilio R, Ronchi D, Hadjigeorgiou GM, Bordoni A, Saladino F, Moggio M, Adobbati L, Kafetsouli D, Tsironi E, Previtali S, Papadimitriou A, Bresolin N, Comi GP. Virgilio R, et al. J Neurol. 2008 Sep;255(9):1384-91. doi: 10.1007/s00415-008-0926-3. Epub 2008 Jun 30. J Neurol. 2008. PMID: 18575922 - Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma.
Filosto M, Mancuso M, Nishigaki Y, Pancrudo J, Harati Y, Gooch C, Mankodi A, Bayne L, Bonilla E, Shanske S, Hirano M, DiMauro S. Filosto M, et al. Arch Neurol. 2003 Sep;60(9):1279-84. doi: 10.1001/archneur.60.9.1279. Arch Neurol. 2003. PMID: 12975295 - [Familial progressive external opthalmoplegia, parkinsonism and polyneuropathy associated with POLG1 mutation].
Mukai M, Sugaya K, Matsubara S, Cai H, Yabe I, Sasaki H, Nakano I. Mukai M, et al. Rinsho Shinkeigaku. 2014;54(5):417-22. doi: 10.5692/clinicalneurol.54.417. Rinsho Shinkeigaku. 2014. PMID: 24943079 Review. Japanese. - Consequences of mutations in human DNA polymerase gamma.
Longley MJ, Graziewicz MA, Bienstock RJ, Copeland WC. Longley MJ, et al. Gene. 2005 Jul 18;354:125-31. doi: 10.1016/j.gene.2005.03.029. Gene. 2005. PMID: 15913923 Review.
Cited by
- Mouse models of oxidative phosphorylation defects: powerful tools to study the pathobiology of mitochondrial diseases.
Torraco A, Diaz F, Vempati UD, Moraes CT. Torraco A, et al. Biochim Biophys Acta. 2009 Jan;1793(1):171-80. doi: 10.1016/j.bbamcr.2008.06.003. Epub 2008 Jun 13. Biochim Biophys Acta. 2009. PMID: 18601959 Free PMC article. Review. - Multi-system neurological disease is common in patients with OPA1 mutations.
Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W Jr, Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF. Yu-Wai-Man P, et al. Brain. 2010 Mar;133(Pt 3):771-86. doi: 10.1093/brain/awq007. Epub 2010 Feb 15. Brain. 2010. PMID: 20157015 Free PMC article. - Secondary mtDNA defects do not cause optic nerve dysfunction in a mouse model of dominant optic atrophy.
Yu-Wai-Man P, Davies VJ, Piechota MJ, Cree LM, Votruba M, Chinnery PF. Yu-Wai-Man P, et al. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4561-6. doi: 10.1167/iovs.09-3634. Epub 2009 May 14. Invest Ophthalmol Vis Sci. 2009. PMID: 19443720 Free PMC article. - Dominant membrane uncoupling by mutant adenine nucleotide translocase in mitochondrial diseases.
Wang X, Salinas K, Zuo X, Kucejova B, Chen XJ. Wang X, et al. Hum Mol Genet. 2008 Dec 15;17(24):4036-44. doi: 10.1093/hmg/ddn306. Epub 2008 Sep 22. Hum Mol Genet. 2008. PMID: 18809618 Free PMC article. - Endocrine manifestations related to inherited metabolic diseases in adults.
Vantyghem MC, Dobbelaere D, Mention K, Wemeau JL, Saudubray JM, Douillard C. Vantyghem MC, et al. Orphanet J Rare Dis. 2012 Jan 28;7:11. doi: 10.1186/1750-1172-7-11. Orphanet J Rare Dis. 2012. PMID: 22284844 Free PMC article. Review.
References
- DiMauro S, Bonilla E. Mitochondrial encephalomyopathies. In: Engel AG, Franzini-Armstrong C, editors. Myology. Vol. 2. New York, NY: McGraw-Hill Co; 2004. pp. 1623–1662.
- Rowland LP. Mitochondrial encephalomyopathies: lumping, splitting, and melding. In: Schapira AHV, DiMauro S, editors. Mitochondrial Disorders in Neurology. Oxford, England: Butterworth-Heinemann; 1994. pp. 116–129.
- Zeviani M, Servidei S, Gellera C, Bertini E, DiMauro S, DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989;339(6222):309–311. - PubMed
- Spinazzola A, Zeviani M. Disorders of nuclear-mitochondrial intergenomic signaling. Gene. 2005;354:162–168. - PubMed
- Kaukonen J, Juselius JK, Tiranti V, et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science. 2000;289(5480):782–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS11766/NS/NINDS NIH HHS/United States
- HD32062/HD/NICHD NIH HHS/United States
- Z01 ES065078/ImNIH/Intramural NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- P01 HD032062/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases