CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain - PubMed (original) (raw)
CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain
Ling Cao et al. Eur J Immunol. 2008 Feb.
Abstract
We previously reported leukocytic infiltration into the lumbar spinal cord in a rodent spinal nerve L5 transection (L5Tx) neuropathic pain model. Here, we further investigated the role of infiltrating T lymphocytes in the etiology of persistent pain following L5Tx. T lymphocyte-deficient nude mice showed no evident mechanical hypersensitivity after day 3 of L5Tx compared to wild-type BALB/c mice. Through FACS analysis, we determined that significant leukocytic infiltration (CD45(hi)) into the lumbar spinal cord peaked at day 7 post L5Tx. These infiltrating leukocytes contained predominantly CD4(+) but not CD8(+) T lymphocytes. B lymphocytes, natural killer cells and macrophages were not detected at day 7 post L5Tx. No differences in the activation of peripheral CD4(+) T lymphocytes were detected in either the spleen or lumbar lymph nodes between L5Tx and sham surgery groups. Further, CD4 KO mice displayed significantly decreased mechanical hypersensitivity after day 7 of L5Tx, and adoptive transfer of CD4(+) leukocytes reversed this effect. Decreased immunoreactivity of glial fibrillary acidic protein observed in CD4 KO mice post L5Tx indicated possible T lymphocyte-glial interactions. These results strongly support a contributing role of spinal cord-infiltrating CD4(+) T lymphocytes versus peripheral CD4(+) T lymphocytes in the maintenance of nerve injury-induced neuropathic pain.
Figures
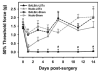
Figure 1. Mechanical sensitivity of nude/BALB/c mice post-L5Tx or sham surgery
Adult male nude/BALB/c mice and BALB/c wild type controls were subjected to L5Tx or sham surgery. Mechanical sensitivity was tested with von Frey filaments using the Up-Down method before and after surgery. Mechanical sensitivity was presented as the 50% threshold force for each animal (n = 8, mean ± SEM). * indicates significant differences between L5Tx and sham operated mice within nude or wild type groups (p < 0.05). ± indicates significant differences between nude and wild type mice subjected to the same surgery (p < 0.05).
Figure 2. Time course of leukocyte infiltration into the lumbar spinal cord post-L5Tx or sham surgery in BALB/c mice
Total lumbar spinal cord cells (from both ipsilateral and contralateral sides) and cells from the cervical region of spinal cord were isolated and labeled with PE-anti-mouse CD45 mAb at day 1, 3, 7 and 14 post sham or L5Tx surgery in adult male BALB/c mice. Representative side scatter (SSC) vs. CD45 plots for total events collected from ipsilateral (ipsi) or contralateral (contra) lumbar spinal cords at day 7 post sham or L5Tx operation (A), naive lumbar spinal cord tissue (left panel in B), and the cervical region of spinal cord day 7 post-L5Tx (right panel in B) are presented. CD45lo and CD45hi indicate populations expressing low and high levels of CD45 respectively. Numbers in the CD45hi region is the average percentage of CD45hi population within total events analyzed (n = 2–5 per group). Time course of CD45hi expression is summarized in C and data are presented as mean ± SEM. “_i_” indicates significant differences between day 7 and day 1, 3 and 14 within ipsilateral L5Tx groups (p < 0.05); and “_c_” indicates significant difference between day 7 and day 14 within contralateral L5Tx groups (p < 0.05)
Figure 3. Phenotype of lumbar spinal cord infiltrating T lymphocytes 7 days post-L5Tx in BALB/c mice
Mononuclear cells were isolated from pooled lumbar spinal cord tissue (3 or 4 mice per group) at day 7 post sham or L5Tx surgery in adult male BALB/c mice. CD45hi population was first identified as shown in A, and then was analyzed in CD3 vs. CD4 dot plots. Results from three independent experiments are presented in B (“n” indicates numbers of animal used for each group; circles indicates CD3+CD4+ population). Similar samples were also obtained from CD4KO mice that underwent L5Tx to ensure the specific identification of CD3+CD4+ T lymphocytes (far right panel in B). Fluorescent immunohistochemical staining for CD4 was performed in the L5 lumbar spinal cord of mice 7 days post-L5Tx. CD4+ cells with typical T lymphocyte morphology were identified mostly in the ipsilateral dorsal horn region. Representative photomicrographs of CD4+ T lymphocytes in the ipsilateral dorsal horn region (arrows) are shown in C (40x, scale bar = 50 μm).
Figure 4. Changes in CD4+ T lymphocytes in the peripheral lymphoid organs post-L5Tx or sham surgery in BALB/c mice
Total cells isolated from spleen and lumbar LNs of sham and L5Tx operated mice were labeled with mAbs and analyzed via FACS. Changes in the numbers of total splenocytes (A), numbers of splenic CD4+ T lymphocytes (CD3+CD4+) (B), and percentage of CD4+ T lymphocytes in the lumbar LNs (C) after surgeries are presented in the left column. Activation of splenic and LNs CD3+CD4+ cells were further determined through their surface expression of CD69, CD154 and CD45RB. Representative data are shown in D–F in the right column. Numbers of animals per group are indicated in each graph. Data are presented as mean ± SEM and “s” or “t” denote significant differences between selected time and day 0 within sham and L5Tx groups, respectively (p < 0.05).
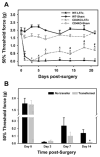
Figure 5. Mechanical sensitivity of CD4KO BALB/c mice post-L5Tx or sham surgery
A: Adult male and female CD4KO BALB/c mice (n = 18 per group) and male BALB/c wild type controls (n = 7 per group) were subjected to L5Tx or sham surgery. Mechanical sensitivity was tested with von Frey filaments using the Up-Down method before and after surgery. Mechanical sensitivity was presented as the 50% threshold force for each animal (mean ± SEM). * indicates significant differences between L5Tx and sham operated mice within CD4KO or wild type groups (p < 0.05). # indicates significant differences between CD4KO and wild type mice subjected to the same surgery (p < 0.05). B: Mechanical sensitivity is shown in CD4 KO mice (black bar, n = 4) and CD4 KO mice adoptively transferred with CD4+ leukocytes (gray bar, n = 6) subjected to L5Tx at different times after surgery. Results of behavioral tests are presented as described in A. * indicates significant differences between non-transferred group and CD4+ leukocyte transferred group (p < 0.05).
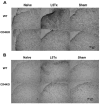
Figure 6. Glial responses in the L5 lumbar spinal cord dorsal horn post-L5Tx or sham surgery in WT and CD4 KO BALB/c mice
L5 lumbar spinal cord sections were prepared from both WT and CD4 KO mice 7 days post-L5Tx or sham surgery and naïve mice (2–3 mice per group). Immunohistochemical staining for GFAP (astrocyte marker, A) and CD11b (microglial marker, B) were performed separately. Representative photomicrographs of the ipsilateral dorsal horn region of L5 lumber spinal cord are shown here (20x, scale bar = 50 μm).
Similar articles
- Anti-nociceptive Role of CXCL1 in a Murine Model of Peripheral Nerve Injury-induced Neuropathic Pain.
Cao L, Malon JT. Cao L, et al. Neuroscience. 2018 Feb 21;372:225-236. doi: 10.1016/j.neuroscience.2017.12.048. Epub 2018 Jan 6. Neuroscience. 2018. PMID: 29309879 Free PMC article. - Phenotypic Identification of Spinal Cord-Infiltrating CD4+ T Lymphocytes in a Murine Model of Neuropathic Pain.
Draleau K, Maddula S, Slaiby A, Nutile-McMenemy N, De Leo J, Cao L. Draleau K, et al. J Pain Relief. 2014 Jun;Suppl 3:003. doi: 10.4172/2167-0846.S3-003. J Pain Relief. 2014. PMID: 25143871 Free PMC article. - Critical role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain.
Cao L, Palmer CD, Malon JT, De Leo JA. Cao L, et al. Eur J Immunol. 2009 Dec;39(12):3562-9. doi: 10.1002/eji.200939657. Eur J Immunol. 2009. PMID: 19750482 Free PMC article. - Contribution of CD137L to Sensory Hypersensitivity in a Murine Model of Neuropathic Pain.
Wakley AA, Leeming R, Malon J, Arabatzis TJ, Yuen Koh W, Cao L. Wakley AA, et al. eNeuro. 2018 Nov 8;5(5):ENEURO.0218-18.2018. doi: 10.1523/ENEURO.0218-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30417077 Free PMC article.
Cited by
- Emerging Role of Serum Glucocorticoid-Regulated Kinase 1 in Pathological Pain.
Liu B, Li N, He Z, Zhang X, Duan G. Liu B, et al. Front Mol Neurosci. 2021 May 20;14:683527. doi: 10.3389/fnmol.2021.683527. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34093127 Free PMC article. - C-X-C Motif Chemokine 10 Contributes to the Development of Neuropathic Pain by Increasing the Permeability of the Blood-Spinal Cord Barrier.
Li HL, Huang Y, Zhou YL, Teng RH, Zhou SZ, Lin JP, Yang Y, Zhu SM, Xu H, Yao YX. Li HL, et al. Front Immunol. 2020 Mar 20;11:477. doi: 10.3389/fimmu.2020.00477. eCollection 2020. Front Immunol. 2020. PMID: 32265928 Free PMC article. - Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents.
Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Ren WJ, et al. Neuropsychopharmacology. 2011 Apr;36(5):979-92. doi: 10.1038/npp.2010.236. Epub 2011 Feb 2. Neuropsychopharmacology. 2011. PMID: 21289602 Free PMC article. - Recent advances in understanding neuropathic pain: glia, sex differences, and epigenetics.
Machelska H, Celik MÖ. Machelska H, et al. F1000Res. 2016 Nov 22;5:2743. doi: 10.12688/f1000research.9621.1. eCollection 2016. F1000Res. 2016. PMID: 28105313 Free PMC article. Review. - Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management.
Vanderwall AG, Milligan ED. Vanderwall AG, et al. Front Immunol. 2019 Dec 23;10:3009. doi: 10.3389/fimmu.2019.03009. eCollection 2019. Front Immunol. 2019. PMID: 31921220 Free PMC article. Review.
References
- Backonja MM. Defining neuropathic pain. Anesth Analg. 2003;97:785–790. - PubMed
- Wang LX, Wang ZJ. Animal and cellular models of chronic pain. Adv Drug Deliv Rev. 2003;55:949–965. - PubMed
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. - PubMed
- Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain. 2002;100:163–170. - PubMed
- DeLeo JA, Winkelstein BA, Rutkowski MD. Society for Neuroscience 32nd Annual Meeting. Orlando, FL: Poster presentation; 2002. The modulatory role of spinal chemokine activation in neuropathic pain.
Publication types
MeSH terms
Substances
Grants and funding
- 5 R01 DA 11276/DA/NIDA NIH HHS/United States
- T32 AI 07363-15/AI/NIAID NIH HHS/United States
- R01 DA011276-13/DA/NIDA NIH HHS/United States
- T32 AI007363-15/AI/NIAID NIH HHS/United States
- R01 DA011276-12/DA/NIDA NIH HHS/United States
- R01 DA011276/DA/NIDA NIH HHS/United States
- T32 AI007363/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous