On the relation between promoter divergence and gene expression evolution - PubMed (original) (raw)
On the relation between promoter divergence and gene expression evolution
Itay Tirosh et al. Mol Syst Biol. 2008.
Abstract
Recent studies have characterized significant differences in the cis-regulatory sequences of related organisms, but the impact of these differences on gene expression remains largely unexplored. Here, we show that most previously identified differences in transcription factor (TF)-binding sequences of yeasts and mammals have no detectable effect on gene expression, suggesting that compensatory mechanisms allow promoters to rapidly evolve while maintaining a stabilized expression pattern. To examine the impact of changes in cis-regulatory elements in a more controlled setting, we compared the genes induced during mating of three yeast species. This response is governed by a single TF (STE12), and variations in its predicted binding sites can indeed account for about half of the observed expression differences. The remaining unexplained differences are correlated with the increased divergence of the sequences that flank the binding sites and an apparent modulation of chromatin structure. Our analysis emphasizes the flexibility of promoter structure, and highlights the interplay between specific binding sites and general chromatin structure in the control of gene expression.
Figures
Figure 1
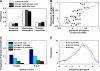
Expression divergence of yeast genes with diverged TF sequence motifs. (A) The percentage of genes with conserved, intermediate or diverged expression among those with conserved or diverged motifs as predicted by Doniger and Fay (2007) and by a similar analysis (see Materials and methods and Supplementary Figure 2). The difference between any pair of the three sets is not statistically significant (_P_>0.05). (B) Average expression divergence for genes with conserved or diverged motifs for various TFs. Some stress-related TFs (e.g. GCN4, DAL82) have relatively high ED of genes with diverged motifs, but in none of these cases it is significantly higher than the respective ED of genes with conserved motifs. (C) Percentage of _S. cerevisiae_-bound promoters at two different binding _P_-values (Harbison et al, 2004) among promoters with different patterns of motif conservation and divergence. The difference between each pair of different patterns is significant (P<0.05). (D) Expression divergence between human and mouse liver cells of genes with conserved, diverged or no binding by four liver-related TFs.
Figure 2
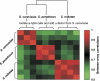
Correlations between the mating expression program in different species. We isolated a-type cells from S. cerevisiae, S. paradoxus and S. mikatae, subjected them to S. cerevisiae α-factor and measured their genome-wide expression profiles using S. cerevisiae arrays. Each species was measured with three or four biological repeats. The correlations among these genomic responses were calculated over 3248 genes with a significant response in at least one experiment.
Figure 3
Differential expression pattern in the mating response. Differentially expressed genes were classified into 12 patterns of up- or downregulation in a subset of the species (see Materials and methods). Each subfigure shows the log2 expression ratios of genes from two classes corresponding to up- and downregulation in a specific subset of species. (A) S. cerevisiae, (B) S. paradoxus, (C) S. mikatae, (D) S. paradoxus+S. mikatae, (E) S. cerevisiae+S. mikatae, (F) S. cerevisiae+S. paradoxus. Cer, par and mik indicate the columns corresponding to expression of S. cerevisiae, S. paradoxus and S. mikatae, respectively. The corresponding subset of species, number of genes within each class, enriched GO annotations and selected genes are indicated at the top of each subfigure. Red and green correspond to up- and downregulation, respectively.
Figure 4
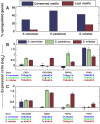
Mating response of genes with conserved or diverged STE12 sequence motifs. (A) The percentage of upregulated genes in response to α-factor is significantly higher among genes whose STE12 sequence motif is conserved (blue) than those whose sequence motifs has diverged (red) in each species (_P_=1 × 10−3, 4 × 10−6, 1 × 10−4 for S. cerevisiae, S. paradoxus and S. mikatae, respectively). Only sequence motifs which are conserved in two species and diverged in another were considered, and these were defined as ‘conserved' and ‘lost' in the respective species (including 27, 17 and 52 genes lost in S. cerevisiae, S. paradoxus and S. mikatae, respectively). Upregulation was defined by log2 ratio>0.5, yet other thresholds gave similar results. (B) Four examples of genes in which divergence of STE12 sequence motifs was associated with reduced response to α-factor. Conserved and mutated STE12 sequence motifs are shown beneath each gene; mutated positions are indicated by black and lowercase. (C) Four examples of genes in which the presence of STE12 sequence motifs is not correlated with the response to α-factor.
Figure 5
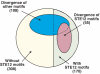
Differential upregulation classified by the presence and divergence of TF-binding sites. Venn diagram showing all the pairwise species differences in upregulation, classified by the presence of STE12 sequence motifs (yellow—no motif, green—motif in at least one of the species), the divergence of STE12 sequence motifs (red—expression differences are consistent with divergence of STE12 sequence motifs) and the divergence of additional TFs which are significantly associated with differences in the mating response (blue—expression differences are consistent with divergence of TF sequence motifs). Numbers in parentheses indicate the number of genes in each class.
Figure 6
Divergence of flanking promoter sequences and chromatin structure. (A) The average conservation of promoter sequences flanking conserved STE12 sequence motifs is shown as a function of the distance from the sequence motif (_x_-axis) and the conservation of the expression response (color). The difference in conservation between genes with unexplained differential expression and the other two gene sets is significant only at the −40 to +40 interval (P<0.05). (B) Maximal interspecies differences in the predicted nucleosome occupancy at the location of conserved STE12-binding sites. (C) Predicted nucleosome occupancy at the regions of conserved STE12-binding sites for the three genes with the largest differences in nucleosome occupancy among those with unexplained differential upregulation (interspecies differences larger than 0.5). The three species are indicated by different colors (blue, purple and orange correspond to cerevisiae, paradoxus and mikatae, respectively). (D) Expression response (log ratio) of the three genes shown in (C). (E) Predicted configuration of STE12 binding (circles) and nucleosomes (ovals) at the STE12-binding sites of the three genes.
Similar articles
- Position specific variation in the rate of evolution in transcription factor binding sites.
Moses AM, Chiang DY, Kellis M, Lander ES, Eisen MB. Moses AM, et al. BMC Evol Biol. 2003 Aug 28;3:19. doi: 10.1186/1471-2148-3-19. Epub 2003 Aug 28. BMC Evol Biol. 2003. PMID: 12946282 Free PMC article. - Evolution of promoter affinity for transcription factors in the human lineage.
Molineris I, Grassi E, Ala U, Di Cunto F, Provero P. Molineris I, et al. Mol Biol Evol. 2011 Aug;28(8):2173-83. doi: 10.1093/molbev/msr027. Epub 2011 Feb 18. Mol Biol Evol. 2011. PMID: 21335606 - A yeast hybrid provides insight into the evolution of gene expression regulation.
Tirosh I, Reikhav S, Levy AA, Barkai N. Tirosh I, et al. Science. 2009 May 1;324(5927):659-62. doi: 10.1126/science.1169766. Science. 2009. PMID: 19407207 - Evolution of transcriptional control in mammals.
Wilson MD, Odom DT. Wilson MD, et al. Curr Opin Genet Dev. 2009 Dec;19(6):579-85. doi: 10.1016/j.gde.2009.10.003. Epub 2009 Nov 11. Curr Opin Genet Dev. 2009. PMID: 19913406 Review. - Improving Estimates of Compensatory cis-trans Regulatory Divergence.
Fraser HB. Fraser HB. Trends Genet. 2019 Jan;35(1):3-5. doi: 10.1016/j.tig.2018.09.003. Epub 2018 Sep 27. Trends Genet. 2019. PMID: 30270122 Free PMC article. Review.
Cited by
- Tracing the evolution of lineage-specific transcription factor binding sites in a birth-death framework.
Yokoyama KD, Zhang Y, Ma J. Yokoyama KD, et al. PLoS Comput Biol. 2014 Aug 21;10(8):e1003771. doi: 10.1371/journal.pcbi.1003771. eCollection 2014 Aug. PLoS Comput Biol. 2014. PMID: 25144359 Free PMC article. - Divergent MLS1 Promoters Lie on a Fitness Plateau for Gene Expression.
Bergen AC, Olsen GM, Fay JC. Bergen AC, et al. Mol Biol Evol. 2016 May;33(5):1270-9. doi: 10.1093/molbev/msw010. Epub 2016 Jan 18. Mol Biol Evol. 2016. PMID: 26782997 Free PMC article. - Gene expression variations are predictive for stochastic noise.
Dong D, Shao X, Deng N, Zhang Z. Dong D, et al. Nucleic Acids Res. 2011 Jan;39(2):403-13. doi: 10.1093/nar/gkq844. Epub 2010 Sep 21. Nucleic Acids Res. 2011. PMID: 20860999 Free PMC article. - Computational analysis of constraints on noncoding regions, coding regions and gene expression in relation to Plasmodium phenotypic diversity.
Essien K, Hannenhalli S, Stoeckert CJ Jr. Essien K, et al. PLoS One. 2008 Sep 1;3(9):e3122. doi: 10.1371/journal.pone.0003122. PLoS One. 2008. PMID: 18769675 Free PMC article. - Positive selection at high temperature reduces gene transcription in the bacteriophage ϕX174.
Brown CJ, Zhao L, Evans KJ, Ally D, Stancik AD. Brown CJ, et al. BMC Evol Biol. 2010 Dec 3;10:378. doi: 10.1186/1471-2148-10-378. BMC Evol Biol. 2010. PMID: 21129199 Free PMC article.
References
- Borneman AR, Gianoulis TA, Zhang ZD, Yu H, Rozowsky J, Seringhaus MR, Wang LY, Gerstein M, Snyder M (2007) Divergence of transcription factor binding sites across related yeast species. Science 317: 815–819 - PubMed
- Dermitzakis ET, Clark AG (2002) Evolution of transcription factor binding sites in mammalian gene regulatory regions: conservation and turnover. Mol Biol Evol 19: 1114–1121 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous