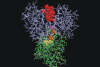A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis - PubMed (original) (raw)
A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis
Cecilia Poderoso et al. PLoS One. 2008.
Abstract
ERK1/2 is known to be involved in hormone-stimulated steroid synthesis, but its exact roles and the underlying mechanisms remain elusive. Both ERK1/2 phosphorylation and steroidogenesis may be triggered by cAMP/cAMP-dependent protein kinase (PKA)-dependent and-independent mechanisms; however, ERK1/2 activation by cAMP results in a maximal steroidogenic rate, whereas canonical activation by epidermal growth factor (EGF) does not. We demonstrate herein by Western blot analysis and confocal studies that temporal mitochondrial ERK1/2 activation is obligatory for PKA-mediated steroidogenesis in the Leydig-transformed MA-10 cell line. PKA activity leads to the phosphorylation of a constitutive mitochondrial MEK1/2 pool with a lower effect in cytosolic MEKs, while EGF allows predominant cytosolic MEK activation and nuclear pERK1/2 localization. These results would explain why PKA favors a more durable ERK1/2 activation in mitochondria than does EGF. By means of ex vivo experiments, we showed that mitochondrial maximal steroidogenesis occurred as a result of the mutual action of steroidogenic acute regulatory (StAR) protein -a key regulatory component in steroid biosynthesis-, active ERK1/2 and PKA. Our results indicate that there is an interaction between mitochondrial StAR and ERK1/2, involving a D domain with sequential basic-hydrophobic motifs similar to ERK substrates. As a result of this binding and only in the presence of cholesterol, ERK1/2 phosphorylates StAR at Ser(232). Directed mutagenesis of Ser(232) to a non-phosphorylable amino acid such as Ala (StAR S232A) inhibited in vitro StAR phosphorylation by active ERK1/2. Transient transfection of MA-10 cells with StAR S232A markedly reduced the yield of progesterone production. In summary, here we show that StAR is a novel substrate of ERK1/2, and that mitochondrial ERK1/2 is part of a multimeric protein kinase complex that regulates cholesterol transport. The role of MAPKs in mitochondrial function is underlined.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
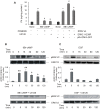
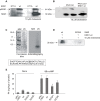
Figure 1. MEK1/2 activation and active ERK1/2 are strictly required for steroidogenesis.
(A) MA-10 cells were pre-incubated for 30 minutes with 50 µM of PD98059 or 10 µM of U0126. Then, they were treated with or without 0.5 mM of 8Br-cAMP for 15 minutes. Progesterone (P4) was measured by radioimmunoassay (RIA) as previously described , (a, p<0.05 vs. control; b, p<0.05 vs. 8Br-cAMP alone). MA-10 cells were also transfected by electroporation using two plasmids, bearing the sequence of wild-type or a mutated form of ERK2 (H230R). Data are expressed as means±SD of three independent experiments. c, p<0.05 vs. control transfection pRc/CMVi-GFP; d, p<0.05 vs. transfected cells+8Br-cAMP; e, p<0.05 vs. wild-type ERK2). After the incubation with or without 10 µM of U0126, MA-10 cells were stimulated with 0.5 mM of 8Br-cAMP (B) or 10 ng/ml of EGF (C) for the indicated times. Cell lysates were subjected to SDS-PAGE and Western blot as previously described , with specific antibodies against pERK1/2 and total ERK1/2 sequentially. The immunoblots show a representative result of three independent experiments. The intensity of the bands was quantitated using total ERK1/2 as loading control. Bars denote relative levels of pERK1/2 presence in arbitrary units. Data are expressed as means±SD of three independent experiments. **, p<0.01 vs. 0 min.
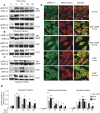
Figure 2. Comparison of subcellular distribution of hormone- or EGF-stimulated pERK1/2 activation.
MA-10 cells were incubated with or without 0.5 mM 8Br-cAMP (upper panels of A, B and C), 10 ng/ml of EGF (middle panels of A, B and C) or 20 ng/ml of hCG (lower panels of A, B and C) for the indicated times. Next, subcellular fractions were obtained and 40 µg of the total protein of each fraction were subjected to SDS-PAGE and Western blot to detect pERK1/2 (indicated with pERK1/2 in A, B, and C). After stripping, total ERK1/2 (indicated with ERK1/2 in A, B, and C) was detected in the same membrane. The Western blots show the results of a representative experiment performed three times. D shows the quantification, performed as described in Figure 1. **, p<0.01, *, p<0.05 vs. time 0. (E) Immunofluorescent staining for pERK1/2 (green) and mitochondria (red) in MA-10 cells after treatment with or without 0.5 mM of 8Br-cAMP or 10 ng/ml of EGF for the indicated times. Merged images are shown in the right panel.
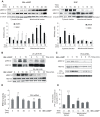
Figure 3. MEK1/2 activation in mitochondria and cytosol is entirely dependent on stimulus type and on PKA activity.
(A) MA-10 cells were stimulated with 0.5 mM 8Br-cAMP or 10 ng/ml of EGF for the indicated times. Cytosolic and mitochondrial pMEK1/2 contents were analyzed by Western blot. A mitochondrial acyl-CoA thioesterase (Acot2), the 39 kDa subunit of the NADH-cytochrome c reductase (complex I) and cytosolic β-tubulin detection were used as loading control. The immunoblots show a representative result of three independent experiments. Bars denote levels of pMEK1/2 (black bars) and total MEK1/2 (grey bars) relative to β-tubulin (cytosolic fractions), Acot2 (mitochondrial fractions- 8Br-cAMP treatments) or complex I (mitochondrial fractions – EGF treatments) in arbitrary units. Data are expressed as means±SD of three independent experiments. * p<0.05 vs. 0 min; **, p<0.01 vs. 0 min. (B) Mitochondrial pERK1/2 and pMEK1/2 contents were analyzed in mitochondria obtained from MA-10 cells stimulated with or without 0.5 mM 8Br-cAMP for varying times, in the presence or absence of 20 µM of H-89, an inhibitor of PKA activity. Acot2 detection was used as loading control in pMEK1/2 and total MEK1/2 western blots. The panel shows a representative immunoblot from three independent experiments. (C) MA-10 cells were transiently transfected with 100 nM siRNA against the α isoform of the PKA catalytic subunit, using Lipofectamine 2000 reagent. After transfection, the cells were incubated with or without 0.5 mM of 8Br-cAMP for varying times and the contents of mitochondrial pMEK1/2, total MEK1/2, α isoform of the PKA catalytic subunit and the 39 kDa subunit of the NADH-cytochrome c reductase (complex I) were analyzed by western blot. This is a representative experiment from three separate experiments. (D) MA-10 cells were transiently transfected as described in (C) and stimulated with 8Br-cAMP for 15 and 30 minutes. pERK1/2 activity was measured using a pERK1/2 (pThr185/pTyr187) ELISA kit (Sigma Chemical Company, St. Louis, MO, USA), following the manufacturer's instructions. Bars represent the pERK1/2 activity as means±SD (n = 3). a, p<0.05, mock-transfected and 8Br-cAMP-treated cells (15 min) vs. mock-transfected and non-8Br-cAMP-treated cells (0 min); b, p<0.05, PKA catalytic subunit siRNA–transfected and 8Br-cAMP-treated (15 min) cells vs. mock-transfected and 8Br-cAMP-treated cells (15 min). (E) PKA activity in cytosolic and mitochondrial fractions isolated from MA-10 cells incubated with 0.5 mM 8Br-cAMP for varying times. Bars represent radioactivity incorporated into the kemptide-specific PKA synthetic substrate. The PKA assay was performed as previously described . Data are expressed as means±SD (n = 3). **, p<0.01, * p<0.05 vs. 0 min.
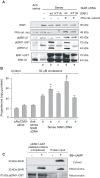
Figure 4. PKA and ERK1/2 are strictly required to achieve maximal progesterone production by isolated mitochondria.
MA-10 cells were transiently transfected by electroporation with an empty vector or with a vector containing StAR cDNA (sense or antisense orientations). Mitochondria were incubated in the presence of cholesterol as substrate (a) in the presence of wild type ERK1-GST protein alone (b) or together with PKA catalytic subunit (d). The mutated inactive form of ERK1-GST (K71A) was also used (c and e). After the indicated incubations, mitochondria were pelleted and subjected to SDS-PAGE and Western blot (A). Specific antibodies that recognize StAR protein, the catalytic subunit of PKA, pMEK1/2, pERK1/2 and total ERK1/2 were used. The panel shows representative Western blots of three independently performed experiments. P4 production is shown in (B). Data are expressed as means±SD (n = 3). * p<0.05 bar b vs. bar a and § p<0.05 bar d vs. bar b. (C) MA-10 cells were treated with or without 0.5 mM of 8Br-cAMP for 3 hours; cytosolic and mitochondrial subcellular fractions were obtained and incubated in the presence or absence of human pERK1-GST bound to agarose beads. Input and pelleted proteins bound to pERK1-GST (complexes) were analyzed by SDS-PAGE and Western blot. The immunoblots show the bands corresponding to StAR and pERK1/2, as loading control. Data are representative of three independently performed experiments.
Figure 5. StAR is phosphorylated in Ser232 by ERK1 in a cholesterol-dependent manner.
Thirty µg of recombinant purified 30 kDa wild-type StAR were incubated in the absence or in the presence of 10 µM of cholesterol (panels A and B) together with constitutively active His-tagged wild type ERK1 (wt) or the mutated inactive form of ERK1 (K71A) (panel A) or together with the catalytic subunit of PKA (cs) in the presence or absence of His-tagged wild type ERK1 (panel B). After the phosphorylation assays, phosphorylated protein levels were analyzed by SDS-PAGE and autoradiography. (C) StAR was phosphorylated by His-tagged wild type ERK1 in the presence of cholesterol as mentioned above. The phosphorylated product was subjected to limited digestion with endoprotease V8 as previously described . Peptides were analyzed by gels as described by Shagger and Von Jagow and by autoradiography. The box indicates the amino acid sequence of the 6 kDa peptide, where the Ser232 is underlined. (D) The wild type and a mutated form (S232A) of StAR were used in the in vitro phosphorylation assay described in (A). The phosphorylated products were analyzed by SDS-PAGE and autoradiography. The results in panels A, B, C, and D are representative of three independently performed experiments. (E) MA-10 cells were transfected by electroporation with an empty vector (pRc/CMVi) or with the vector containing the full length cDNAs of 37 kDa wild type StAR (wt), S232A StAR (S232A) or S232E StAR (S232E). The cells were stimulated with 0.5 mM of 8Br-cAMP for 30 minutes. P4 production was evaluated by determination of P4 concentrations in the incubation media by RIA. Data are expressed as means±SD (n = 3). *, p<0.05 S232A StAR vs. wt StAR.
Figure 6. Predictive model of the molecular interaction between ERK1/2 and StAR.
The reconstruction of molecular interaction of ERK2 and StAR was performed using PyMOL (DeLano Scientific, USA;
). ERK2 (Protein Data Bank code 2GPH) is represented in blue and StAR (START domain in StartD4 from Mus musculus) in green. In this model, StAR is located in the docking groove of ERK2. The active center of ERK2 is in dark red. The CD domain of ERK2, represented in yellow, includes Asp316 and Asp319 in contact with the D domain of StAR. Lys174 and Lys176, corresponding to Lys235 and Lys237 of StAR sequence in Mus musculus are represented in orange, and Ser171, corresponding to Ser232 of murine StAR, in dark pink.
Similar articles
- Hormonal activation of a kinase cascade localized at the mitochondria is required for StAR protein activity.
Poderoso C, Maloberti P, Duarte A, Neuman I, Paz C, Cornejo Maciel F, Podesta EJ. Poderoso C, et al. Mol Cell Endocrinol. 2009 Mar 5;300(1-2):37-42. doi: 10.1016/j.mce.2008.10.009. Epub 2008 Oct 19. Mol Cell Endocrinol. 2009. PMID: 19007846 Review. - cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation.
Manna PR, Chandrala SP, Jo Y, Stocco DM. Manna PR, et al. J Mol Endocrinol. 2006 Aug;37(1):81-95. doi: 10.1677/jme.1.02065. J Mol Endocrinol. 2006. PMID: 16901926 - Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse leydig tumor cells.
Dyson MT, Jones JK, Kowalewski MP, Manna PR, Alonso M, Gottesman ME, Stocco DM. Dyson MT, et al. Biol Reprod. 2008 Feb;78(2):267-77. doi: 10.1095/biolreprod.107.064238. Epub 2007 Nov 7. Biol Reprod. 2008. PMID: 17989356 - Molecular control of luteal secretion of progesterone.
Niswender GD. Niswender GD. Reproduction. 2002 Mar;123(3):333-9. doi: 10.1530/rep.0.1230333. Reproduction. 2002. PMID: 11882010 Review.
Cited by
- SCAP/SREBP pathway is required for the full steroidogenic response to cyclic AMP.
Shimizu-Albergine M, Van Yserloo B, Golkowski MG, Ong SE, Beavo JA, Bornfeldt KE. Shimizu-Albergine M, et al. Proc Natl Acad Sci U S A. 2016 Sep 20;113(38):E5685-93. doi: 10.1073/pnas.1611424113. Epub 2016 Sep 6. Proc Natl Acad Sci U S A. 2016. PMID: 27601673 Free PMC article. - Long-lasting spinal oxytocin analgesia is ensured by the stimulation of allopregnanolone synthesis which potentiates GABA(A) receptor-mediated synaptic inhibition.
Juif PE, Breton JD, Rajalu M, Charlet A, Goumon Y, Poisbeau P. Juif PE, et al. J Neurosci. 2013 Oct 16;33(42):16617-26. doi: 10.1523/JNEUROSCI.3084-12.2013. J Neurosci. 2013. PMID: 24133265 Free PMC article. - Hormone-dependent expression of a steroidogenic acute regulatory protein natural antisense transcript in MA-10 mouse tumor Leydig cells.
Castillo AF, Fan J, Papadopoulos V, Podestá EJ. Castillo AF, et al. PLoS One. 2011;6(8):e22822. doi: 10.1371/journal.pone.0022822. Epub 2011 Aug 1. PLoS One. 2011. PMID: 21829656 Free PMC article. - Inhibition of testosterone synthesis induced by oral TiO2 NPs is associated with ROS-MAPK(ERK1/2)-StAR signaling pathway in SD rat.
Liu S, Tang Y, Chen B, Zhao Y, Aguilar ZP, Tao X, Xu H. Liu S, et al. Toxicol Res (Camb). 2021 Aug 9;10(4):937-946. doi: 10.1093/toxres/tfab077. eCollection 2021 Aug. Toxicol Res (Camb). 2021. PMID: 34484685 Free PMC article. - VLDL-activated cell signaling pathways that stimulate adrenal cell aldosterone production.
Tsai YY, Rainey WE, Johnson MH, Bollag WB. Tsai YY, et al. Mol Cell Endocrinol. 2016 Sep 15;433:138-46. doi: 10.1016/j.mce.2016.05.018. Epub 2016 May 21. Mol Cell Endocrinol. 2016. PMID: 27222295 Free PMC article.
References
- Crivello JF, Jefcoate CR. Intracellular movement of cholesterol in rat adrenal cells. Kinetics and effects of inhibitors. J Biol Chem. 1980;255:8144–8151. - PubMed
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. - PubMed
- Liu J, Rone MB, Papadopoulos V. Protein-Protein Interactions Mediate Mitochondrial Cholesterol Transport and Steroid Biosynthesis. J Biol Chem. 2006;281:38879–38893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous