Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage - PubMed (original) (raw)
Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage
Yechiel Elkabetz et al. Genes Dev. 2008.
Erratum in
- Genes Dev. 2008 May 1;22(9):1257
Abstract
Neural stem cells (NSCs) yield both neuronal and glial progeny, but their differentiation potential toward multiple region-specific neuron types remains remarkably poor. In contrast, embryonic stem cell (ESC) progeny readily yield region-specific neuronal fates in response to appropriate developmental signals. Here we demonstrate prospective and clonal isolation of neural rosette cells (termed R-NSCs), a novel NSC type with broad differentiation potential toward CNS and PNS fates and capable of in vivo engraftment. R-NSCs can be derived from human and mouse ESCs or from neural plate stage embryos. While R-NSCs express markers classically associated with NSC fate, we identified a set of genes that specifically mark the R-NSC state. Maintenance of R-NSCs is promoted by activation of SHH and Notch pathways. In the absence of these signals, R-NSCs rapidly lose rosette organization and progress to a more restricted NSC stage. We propose that R-NSCs represent the first characterized NSC stage capable of responding to patterning cues that direct differentiation toward region-specific neuronal fates. In addition, the R-NSC-specific genetic markers presented here offer new tools for harnessing the differentiation potential of human ESCs.
Figures
Figure 1.
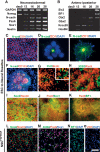
hESC-derived neural rosettes adopt polarized neuroepithelial structures of anterior CNS fate. (A) RT–PCR analysis during neural rosette formation for neurectodermal (Pax6 and Sox1), neuroepithelial (N-cad), and neural precursor (Nestin) markers, as well as markers of undifferentiated hESCs (Nanog). (B) RT–PCR analysis for anterior CNS markers (BF1, Six3, and Otx2) and markers of posterior CNS fate (Gbx2, Krox20, and Hoxb4). (C) Immunocytochemistry for tight junction protein ZO-1 in undifferentiated hESCs, in day 12 rosettes (D), and in day 16 rosettes colocalized with N-cad (E). (F) Immunocytochemistry for CD133 and N-cad. Dashed line outlines a single rosette with an average diameter of ∼100 μm. (G) Immunocytochemistry for PH3 and BrdU. Evidence of interkinetic nuclear migration. (H) Expression of Pax6 and the radial glia marker 3CB2. Immunocytochemistry in hESC-derived rosettes for markers of NSCs (Nestin and Sox2; I), neuroepithelial cells (Pax6 and Sox1; J), and anterior fate (Pax6 and BF1; K). Immunocytochemistry in NSCsFGF2/EGF for markers of NSCs (Nestin and Sox2; L), radial glia and neuroepithelial cells (Pax6, 3CB2, and Sox1; M), and anterior fate (BF1; N, and ZO-1; O). The morphological progression to rosette and NSCsFGF2/EGF state is presented in Supplemental Figure 1. Bar in O corresponds to 12.5 μm in D, 35 μm in G (inset), 50 μm in E and H, 67 μm in C and G, 100 μm in I and L_–_N, 125 μm in F, and 200 μm in J and K.
Figure 2.
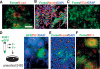
Forse1 is a novel NSC marker for the prospective isolation of anterior neural rosette cells (A). Immunocytochemistry for Forse1 in N-cad+ rosettes. Forse1 and Nestin (NSC marker) (B) and Forse1 and β3-tubulin (neuronal marker) (C) staining in dissociated rosette stage cells. (D, left panel) Forse1+/N-cadhigh rosette stage cells were isolated from RU-01eGFP and replated at single-cell density on stage-matched high-cell-density rosette cells from H9 (WA-09). (Right panel) Representative image showing clonally derived ZO-1+/eGFP+ rosette. (E) Immunocytochemistry for Forse1 and N-cad demonstrates the presence of Forse1+ and Forse1− rosettes. (F) Forse1 colocalization with the telencephalic marker BF1. Bar in A corresponds to 50 μm in A_–_C and E, 75 μm in D, and 100 μm in F.
Figure 3.
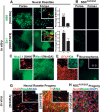
Respecification of anterior neural rosettes toward caudal fates. (A) Analysis and quantification of HB9+ motoneurons and En1+ midbrain precursors in response to SHH/RA- or SHH/FGF8-mediated patterning, respectively, of Forse1+ and Forse1− rosette stage cells. Insets show representative images following clonal analyses for GFP+ Forse1+ rosette stage cells exposed to SHH/RA or SHH/FGF8, respectively. Top inset) HB9 (red) and GFP (green). (Bottom inset) En1 (red) and GFP (green). Statistical analysis: mean ± SEM; (**) P < 0.01; (*) P < 0.05; ANOVA. (B) Results for SHH/RA- or SHH/FGF8-treated Forse1+ and Forse1− NSCsFGF2/EGF showing a complete lack of HB9+ motoneurons and near complete lack of En1+ precursors. (C) Immunocytochemistry for the ventral forebrain marker Nkx2.1 upon exposure of Forse1+ rosette stage cells to SHH. (Inset) Prolonged differentiation yielded cells immunoreactive for GABA (red). (D) Induction of the dorsal marker Msx1 was observed upon exposure of Forse1+ rosette stage cells to Wnt3A. (E) Analysis for GFAP and O4 (inset) expression in rosette stage cells expanded in FGF2/EGF followed by exposure to CNTF or T3, respectively. (F) Neurosphere formation of Forse1+ R-NSCs and Forse1− R-NSCs (inset; note differences in size). (G) In vivo survival of spinal motoneurons and midbrain dopamine neurons derived from rosettes in vitro. Insets show confocal image stacks after 3D reconstruction confirming human identity of the grafted cells. (H) Expression of neuronal markers in NSCFGF2/EGF progeny in vivo. Bar in A, (top right panel) corresponds to 100 μm in A and B (top panels), and D, E, G, and H; 200 μm in C; 250 μm in A and B (bottom panels); and 500 μm in F.
Figure 4.
Genetic characterization of R-NSCs and NSCsFGF2/EGF. (A) Global gene expression analysis (Affymetrix, U133-Plus2) comparing Forse1+ versus Forse1− R-NSCs at P1 (day 25). (B) Venn diagram representing genes specifically expressed in Forse1+ (red) and Forse1− (green) R-NSCs, and in Forse1+ NSCsFGF2/EGF (blue). R-NSC-specific genes are marked in yellow, while genes shared between all three NSC groups are marked in white. (C) Fold changes calculated from Affymetrix analysis for the top differentially expressed transcripts. Fold changes are grouped into those specific for R-NSCs, those shared between R-NSCs and NSCsFGF2/EGF, and those specific to NSCsFGF2/EGF. (D) Immunocytochemical confirmation of representative markers shared between R-NSCs and NSCsFGF2/EGF (Zic1), or markers specific to R-NSC state (Dach1). (E) Colabeling studies of 3CB2 and NSCsFGF2/EGF-specific (AQP4) or R-NSC-specific (PLZF) markers. Bar in D corresponds to 50 μm for all panels.
Figure 5.
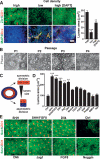
Effects of cell density, SHH, and Notch signaling pathways on R-NSC maintenance. (A) Effect of cell plating density and DAPT treatment on rosette reformation (Zic/ZO-1) and spontaneous neuronal differentiation (MAP2/Dcx) in dissociated P2 R-NSCs. Right panel shows quantification of rosette lumens as a surrogate marker of rosette growth. Statistical analysis: mean ± SEM; (***) P < 0.001; (**) P < 0.01 (compared with high density; ANOVA: Newman-Keuls test). (B) Phase contrast images of R-NSCs maintained in SHH/FGF8 over multiple passages (loss of rosette structure). (C) Schematic model illustrating symmetric versus asymmetric division mode in rosettes and relationship to lumen size. (D) Quantification of ZO-1+ rosette lumen size in P2 R-NSC cultures treated with various agonists and antagonists of candidate signaling pathways. Statistical analysis: mean ± SEM; (***) P < 0.001; (**) P < 0.01 (compared with control; ANOVA: Dunnett test). (E) Representative images (ZO-1/Sox1) of P2 R-NSC cultures at day 4 of treatment with candidate factors. Scale bar in A corresponds to 50 μm in A and B, and 75 μm in E.
Figure 6.
R-NSC maintenance and transition to NSCsFGF2/EGF stage. Representative phase-contrast images (A) and growth curves (B) over multiple passages in R-NSC cultures maintained with candidate growth factor regimens. (C) Corresponding immunocytochemical analyses for rosette structure (Zic1/ZO-1), markers of R-NSCs (PLZF), NSCsFGF2/EGF (S100B), and radial glia (3CB2). (D) Clonal analysis of constitutively eGFP-expressing R-NSCs sorted for Forse1. Cells are shown at the transition toward NSCsFGF/EGF with partial loss of PLZF expression and loss of epithelial organization in a subset of clonally derived eGFP+ cells. (E) Effect of passage and growth regimen on “rosette score,” a composite measure for gene expression for all 298 R-NSC markers. (F) qRT–PCR analysis for PLZF expression over passage in SHH/Dll4/Jag- versus SHH/FGF8-treated R-NSCs. Bar in C corresponds to 50 μm in A and C.
Figure 7.
Generalization of neural rosette biology: in vivo maintenance, derivation from mouse ESCs and from primary neural plate tissue. (A) In vivo behavior of hESC-derived rosettes: immunohistochemical analysis of R-NSC progeny 4 wk after transplantation into the adult rat striatum. (B) Characterization of mouse ESC-derived neural rosettes: Immunocytochemical analysis is shown for rosette structure (ZO-1,DAPI), R-NSC marker (PLZF, Dach1), and anterior fate (BF1). (C) Neural rosettes derived from primary mouse neural plate: Anterior neural plate tissue from E8.25 mouse embryos was maintained for 4 d in the presence of SHH/Dll4/Jag. Emerging rosettes were characterized for rosette structure (phase-contrast image, ZO-1) and R-NSC marker expression (Dach1, PLZF). Bar in A corresponds to 50 μm in C (middle and right panels); 100 μm in A (middle and right panels), B (all panels), and C (left panel); and 150 μm in A (left panel). (D) Model of neural rosette biology: R-NSCs are proposed to represent a novel NSC stage intermediate between undifferentiated ESCs and FGF2/EGF-expanded NSCs (NSCsFGF2/EGF) with distinct marker expression, morphology, fate potential, in vivo behavior, and signaling requirements (see the text for details).
Similar articles
- Human ESC-derived neural rosettes and neural stem cell progression.
Elkabetz Y, Studer L. Elkabetz Y, et al. Cold Spring Harb Symp Quant Biol. 2008;73:377-87. doi: 10.1101/sqb.2008.73.052. Epub 2009 Feb 9. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19204067 Review. - Expression profile of an operationally-defined neural stem cell clone.
Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA, Snyder EY. Parker MA, et al. Exp Neurol. 2005 Aug;194(2):320-32. doi: 10.1016/j.expneurol.2005.04.018. Exp Neurol. 2005. PMID: 15992799 - Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo.
Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O. Borghese L, et al. Stem Cells. 2010 May;28(5):955-64. doi: 10.1002/stem.408. Stem Cells. 2010. PMID: 20235098 - An epigenetic signature of developmental potential in neural stem cells and early neurons.
Burney MJ, Johnston C, Wong KY, Teng SW, Beglopoulos V, Stanton LW, Williams BP, Bithell A, Buckley NJ. Burney MJ, et al. Stem Cells. 2013 Sep;31(9):1868-80. doi: 10.1002/stem.1431. Stem Cells. 2013. PMID: 23712654 - Novel roles for Notch, Wnt and Hedgehog in hematopoesis derived from human pluripotent stem cells.
Cerdan C, Bhatia M. Cerdan C, et al. Int J Dev Biol. 2010;54(6-7):955-63. doi: 10.1387/ijdb.103067cc. Int J Dev Biol. 2010. PMID: 20336604 Review.
Cited by
- Sequencing-based study of neural induction of human dental pulp stem cells.
Takaoka S, Uchida F, Ishikawa H, Toyomura J, Ohyama A, Matsumura H, Hirata K, Fukuzawa S, Kanno NI, Marushima A, Yamagata K, Yanagawa T, Matsumaru Y, Ishikawa E, Bukawa H. Takaoka S, et al. Hum Cell. 2024 Nov;37(6):1638-1648. doi: 10.1007/s13577-024-01121-7. Epub 2024 Aug 29. Hum Cell. 2024. PMID: 39210197 - Emerging strategies for nerve repair and regeneration in ischemic stroke: neural stem cell therapy.
Wang S, He Q, Qu Y, Yin W, Zhao R, Wang X, Yang Y, Guo ZN. Wang S, et al. Neural Regen Res. 2024 Nov 1;19(11):2430-2443. doi: 10.4103/1673-5374.391313. Epub 2023 Dec 21. Neural Regen Res. 2024. PMID: 38526280 Free PMC article. - Stable and efficient generation of functional iPSC-derived neural progenitor cell rosettes through regulation of collective cell-cell behavior.
Kim MH, Thanuthanakhun N, Kino-Oka M. Kim MH, et al. Front Bioeng Biotechnol. 2024 Jan 10;11:1269108. doi: 10.3389/fbioe.2023.1269108. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38268936 Free PMC article. - Zbtb16 mediates a switch between Fgf signalling regimes in the developing hindbrain.
Leino SA, Constable SCJ, Streit A, Wilkinson DG. Leino SA, et al. Development. 2023 Sep 15;150(18):dev201319. doi: 10.1242/dev.201319. Epub 2023 Sep 14. Development. 2023. PMID: 37642135 Free PMC article. - Collective behavior and self-organization in neural rosette morphogenesis.
Miotto M, Rosito M, Paoluzzi M, de Turris V, Folli V, Leonetti M, Ruocco G, Rosa A, Gosti G. Miotto M, et al. Front Cell Dev Biol. 2023 Aug 10;11:1134091. doi: 10.3389/fcell.2023.1134091. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37635866 Free PMC article. Review.
References
- Alvarez Buylla A., Garcia-Verdugo J.M., Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. - PubMed
- Androutsellis-Theotokis A., Leker R.R., Soldner F., Hoeppner D.J., Ravin R., Poser S.W., Rueger M.A., Bae S.K., Kittappa R., McKay R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Barberi T., Klivenyi P., Calingasan N.Y., Lee H., Kawamata H., Loonam K., Perrier A.L., Bruses J., Rubio M.E., Topf N., et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in Parkinsonian mice. Nat. Biotechnol. 2003;21:1200–1207. - PubMed
- Barberi T., Bradbury M., Dincer Z., Panagiotakos G., Socci N.D., Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 2007;13:642–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources