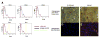Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) - PubMed (original) (raw)
Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs)
Zhaodi Gong et al. FASEB J. 2008 Jun.
Abstract
Using biodegradable scaffold and a biomimetic perfusion system, our lab has successfully engineered small-diameter vessel grafts using endothelial cells (ECs) and smooth muscle cells (SMCs) obtained from vessels in various species. However, translating this technique into humans has presented tremendous obstacles due to species and age differences. SMCs from elderly persons have limited proliferative capacity and a reduction in collagen production, which impair the mechanical strength of engineered vessels. As an alternative cell source, adult human bone marrow-derived mesenchymal stem cells (hMSCs) were studied for their ability to differentiate into SMCs in culture plates as well as in a bioreactor system. In the former setting, immunofluorescence staining showed that MSCs, after induction for 14 days, expressed smooth muscle alpha-actin (SMA) and calponin, early and mid-SMC phenotypic markers, respectively. In the latter setting, vessel walls were constructed with MSC-derived SMCs. Various factors (i.e., matrix proteins, soluble factors, and cyclic strain) in the engineering system were further investigated for their effects on hMSC cell proliferation and differentiation into SMCs. Based on a screening of multiple factors, the engineering system was optimized by dividing the vessel culture into proliferation and differentiation phases. The vessel walls engineered under the optimized conditions were examined histologically and molecularly, and found to be substantially similar to native vessels. In conclusion, bone marrow-derived hMSCs can serve as a new cell source of SMCs in vessel engineering. Optimization of the culture conditions to drive SMC differentiation and matrix production significantly improved the quality of the hMSC-derived engineered vessel wall.
Figures
Figure 1
Confirmation of hMSC phenotype. A) Representative flow cytometric analysis showing reactivity of SH2 and SH3 antibody with hMSCs isolated from human bone marrow (green curve). hMSCs were negative with CD14, CD34, and CD45 antibody (green curve). Staining with the isotype-matched control antibody is shown in blue; cells-only controls are shown in red. B–E) Osteogenic and adipogenic induction of hMSC culture was performed. Osteogenesis was examined 3 wk after treatment in control (B) or induction (C) cultures by ALP and von Kossa staining. After three complete cycles of induction and maintenance, the extent of adipogenesis was examined by Nile red staining of control (D) and induction (E) cultures.
Figure 2
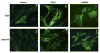
SMC induction in hMSC culture. hMSCs were grown in basal DMEM medium on glass chamber slides without (a, d) or with (b, e) the addition of 1 ng/ml TGFβ1. Immnofluorescence staining (green) was performed for SMA (a–c) and calponin (e, f) on day 14. Nuclei were stained with DAPI (blue). CASMCs were stained for SMA (c) and calponin (f) as positive controls.
Figure 3
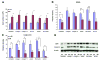
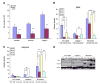
Effect of various matrices on hMSC cell proliferation and differentiation. hMSCs were seeded at 2 × 104/ml in DMEM plus 10% FBS medium on 6-well plates untreated or coated with collagen type I, IV, elastin, fibronectin, and laminin in the presence or absence of 10 ng/ml PDGF-BB. A) After 7 days, cell numbers were counted with methylene blue on a hemacytometer for cell proliferation (**P<0.05, PDGF-BB treatment vs. no BB; no difference between each matrix and untreated with or without PDGF-BB; _n_=5). B, C) Western blots were performed on the protein lysates from each treatment group on SMA (B) and calponin (C, *P<0.05 vs. untreated; **P<0.05, PDGF-BB vs. no BB; _n_=3). D) Representative Western blot on SMA, calponin, and β-actin.
Figure 4
Effect of various factors on hMSC cell proliferation and differentiation. hMSCs were seeded at 5.6 × 103/cm2 in DMEM plus 5% FBS medium with one of the following supplements: 0 (control), 0.01, 0.1, 1, or 10 ng/ml TGFβ1; 10 ng/ml PDGF-BB, PDGF-CC, bFGF; or 50 μg/ml ascorbic acid. A) After 7 days, the cells were enumerated with 3% acetic acid with methylene blue on a hemacytometer (**P<0.05 vs. control; _n_=5). B, C) Western blots were performed on the protein lysates from each treatment group on SMA (B) and calponin (C; **P<0.05 compared to control, _n_=3). D) Representative Western blot on SMA, calponin, and β-actin. E) Immunofluorescence staining for SMA in hMSC culture after treatment with TGFβ1, PDGF-BB, PDGF-CC, bFGF, and vitamin C in addition to control for 7 days. Concentration of each factor is labeled in the figure.
Figure 5
Effect of cyclic strain on hMSC cell proliferation and differentiation. hMSCs were cultured in 6-well plates noncoated or coated with collagen I or fibronectin. In each surface treatment group, there were four culture conditions: control (S− B−), PDGF-BB only (10 ng/ml, S− B+), cyclic strain only (S+ B−), and PDGF plus cyclic strain (S+ B+). A–C) Cells were subject to the detailed condition for 5 days and harvested for cell number (A) and Western blot on SMA (B) and calponin (C). D) Representative Western blot film (_n_=3).
Figure 6
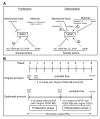
A) Effects of various soluble factors, matrix proteins, and cyclic strain on hMSC cell proliferation and differentiation. B) Optimization of the engineering system to grow a vessel wall using hMSCs.
Figure 7
Representative histological stainings on engineered vessel walls before (a_–_d, i) and after (e_–_h, j) the optimization of engineering conditions: H&E (a, c, e, g), Masson's Trichrome (b, d, f, h), PCNA (i, j). Scale bar is shown in each picture.
Figure 8
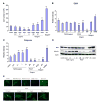
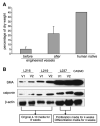
Collagen and Western blot analysis on engineered vessels. A) Collagen assay on the engineered vessel walls before (_n_=6) and after optimization (_n_=4). Human umbilical artery served as positive control. B) Western blot on protein lysate from six engineered human vessels for SMA and calponin. L216 v1 and v2, L219v1 and v2, and L237 v1 and v2 were the pairs of vessels grown in the same bioreactor from the same donor-derived MSCs. Lysates from CASMCs were used as positive control. β-Actin served as equal loading control.
Figure 9
SEM images of the luminal surface of nonendothelialized (A) and endothelialized (B) vessels engineered from hMSCs using the optimized protocol. Scale bars = 50 μm.
Similar articles
- A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells.
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y, Cui L. Wang C, et al. Biomaterials. 2010 Feb;31(4):621-30. doi: 10.1016/j.biomaterials.2009.09.086. Epub 2009 Oct 12. Biomaterials. 2010. PMID: 19819545 - Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering.
Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M, Ueda Y. Suzuki S, et al. Cells Tissues Organs. 2010;191(4):269-80. doi: 10.1159/000260061. Epub 2009 Nov 19. Cells Tissues Organs. 2010. PMID: 19940434 - A study of a three-dimensional PLGA sponge containing natural polymers co-cultured with endothelial and mesenchymal stem cells as a tissue engineering scaffold.
Shim JB, Ankeny RF, Kim H, Nerem RM, Khang G. Shim JB, et al. Biomed Mater. 2014 Aug;9(4):045015. doi: 10.1088/1748-6041/9/4/045015. Epub 2014 Jul 25. Biomed Mater. 2014. PMID: 25065725 - Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain.
Kurpinski K, Park J, Thakar RG, Li S. Kurpinski K, et al. Mol Cell Biomech. 2006 Mar;3(1):21-34. Mol Cell Biomech. 2006. PMID: 16711069 Review. - Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels.
Chan-Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, Kang GC, Greisler HP. Chan-Park MB, et al. J Biomed Mater Res A. 2009 Mar 15;88(4):1104-21. doi: 10.1002/jbm.a.32318. J Biomed Mater Res A. 2009. PMID: 19097157 Review.
Cited by
- Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates.
Bajpai VK, Mistriotis P, Loh YH, Daley GQ, Andreadis ST. Bajpai VK, et al. Cardiovasc Res. 2012 Dec 1;96(3):391-400. doi: 10.1093/cvr/cvs253. Epub 2012 Aug 31. Cardiovasc Res. 2012. PMID: 22941255 Free PMC article. - Proteomic Profiling of Mesenchymal Stem Cell Responses to Mechanical Strain and TGF-beta1.
Kurpinski K, Chu J, Wang D, Li S. Kurpinski K, et al. Cell Mol Bioeng. 2009 Dec;2(4):606-614. doi: 10.1007/s12195-009-0090-6. Epub 2009 Oct 24. Cell Mol Bioeng. 2009. PMID: 20037637 Free PMC article. - Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells.
Han J, Liu JY, Swartz DD, Andreadis ST. Han J, et al. Cardiovasc Res. 2010 Jul 1;87(1):147-55. doi: 10.1093/cvr/cvq024. Epub 2010 Jan 22. Cardiovasc Res. 2010. PMID: 20097675 Free PMC article. - The Role of Sclerostin in Bone and Ectopic Calcification.
Maré A, D'Haese PC, Verhulst A. Maré A, et al. Int J Mol Sci. 2020 Apr 30;21(9):3199. doi: 10.3390/ijms21093199. Int J Mol Sci. 2020. PMID: 32366042 Free PMC article. Review. - Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model.
Koobatian MT, Row S, Smith RJ Jr, Koenigsknecht C, Andreadis ST, Swartz DD. Koobatian MT, et al. Biomaterials. 2016 Jan;76:344-58. doi: 10.1016/j.biomaterials.2015.10.020. Epub 2015 Oct 14. Biomaterials. 2016. PMID: 26561932 Free PMC article.
References
- Niklason LE. Replacement arteries made to order. Science. 1999;286:1493–1494. - PubMed
- Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, Towne JB, Bernhard VM, Bonier P, Flinn WR, Astelford P, Yao JST, Bergan JJ. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104–114. - PubMed
- Chard RB, Johnson DC, Num GR, Cartmill TB. Aorta-coronary bypass grafting with polytetrafluoroethylene conduits. Early and late outcome in eight patients. J Thorac Cardiovasc Surg. 1987;94:132–134. - PubMed
- Sapsford RN, Oakley GD, Talbor S. Early and late patency of expanded polytetrafluoroethylene vascular grafts in aorta-coronary bypass. J Thorac Cardiovasc Surg. 1981;81:860–864. - PubMed
- Steinthorsson G, Sumpio B. Clinical and biological relevance of vein cuff anastomosis. Acta Chir Belg. 1999;99:282–288. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL083895/HL/NHLBI NIH HHS/United States
- R01 HL083895-03/HL/NHLBI NIH HHS/United States
- HL063766/HL/NHLBI NIH HHS/United States
- R01HL083895/HL/NHLBI NIH HHS/United States
- R01 HL063766-05/HL/NHLBI NIH HHS/United States
- R01 HL063766/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous