The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy - PubMed (original) (raw)
. 2008 Jan 16;28(3):737-48.
doi: 10.1523/JNEUROSCI.2824-07.2008.
Astrid Nissen, Katrin Eckermann, Inna Khlistunova, Jacek Biernat, Dagmar Drexler, Olga Petrova, Kai Schönig, Hermann Bujard, Eckhard Mandelkow, Lepu Zhou, Gabriele Rune, Eva-Maria Mandelkow
Affiliations
- PMID: 18199773
- PMCID: PMC6670355
- DOI: 10.1523/JNEUROSCI.2824-07.2008
The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy
Maria-Magdalena Mocanu et al. J Neurosci. 2008.
Abstract
We describe two new transgenic mouse lines for studying pathological changes of Tau protein related to Alzheimer's disease. They are based on the regulatable expression of the four-repeat domain of human Tau carrying the FTDP17 (frontotemporal dementia and parkinsonism linked to chromosome 17) mutation deltaK280 (Tau(RD)/deltaK280), or the deltaK280 plus two proline mutations in the hexapeptide motifs (Tau(RD)/deltaK280/I277P/I308P). The deltaK280 mutation accelerates aggregation ("proaggregation mutant"), whereas the proline mutations inhibit Tau aggregation in vitro and in cell models ("antiaggregation mutant"). The inducible transgene expression was driven by the forebrain-specific CaMKIIalpha (calcium/calmodulin-dependent protein kinase IIalpha) promoter. The proaggregation mutant leads to Tau aggregates and tangles as early as 2-3 months after gene expression, even at low expression (70% of endogenous mouse Tau). The antiaggregation mutant does not aggregate even after 22 months of gene expression. Both mutants show missorting of Tau in the somatodendritic compartment and hyperphosphorylation in the repeat domain [KXGS motifs, targets of the kinase MARK (microtubule affinity regulating kinase)]. This indicates that these changes are related to Tau expression rather than aggregation. The proaggregation mutant causes astrogliosis, loss of synapses and neurons from 5 months of gene expression onward, arguing that Tau toxicity is related to aggregation. Remarkably, the human proaggregation mutant Tau(RD) coaggregates with mouse Tau, coupled with missorting and hyperphosphorylation at multiple sites. When expression of proaggregation Tau(RD) is switched off, soluble and aggregated exogenous Tau(RD) disappears within 1.5 months. However, tangles of mouse Tau, hyperphosphorylation, and missorting remain, suggesting an extended lifetime of aggregated wild-type Tau once a pathological conformation and aggregation is induced by a proaggregation Tau species.
Figures
Figure 1.
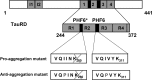
Constructs of Tau derived from Tau repeat domain. The top bar diagram represents the longest isoform of the human tau40 (441 residues). The bottom diagram shows the four-repeat construct TauRD derived from htau40. One of the constructs used here contains the FTDP17 mutation ΔK280 to accelerate aggregation by promoting β-structure (proaggregation mutant). The second mutant has additionally two proline mutations (I277P and I308P in the hexapeptide motifs) to inhibit the aggregation by disrupting β-structure (antiaggregation mutant).
Figure 2.
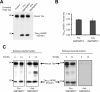
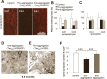
Mutant TauRD protein levels in transgenic mice. Representative immunoblots probed with K9JA polyclonal antibody that recognizes the repeats plus C-terminal domain of Tau. A, Levels of Tau in cortex of mutant TauRD-inducible transgenic mice. Cortex brain lysates from nontransgenic littermate, proaggregation (TauRD/ΔK280) and antiaggregation (TauRD/ΔK280/2P) mutants at 9 months of gene expression. Ten micrograms of total protein is loaded per lane. B, Ratio of mutant TauRD versus endogenous Tau in transgenic mice at 9 months of gene expression (n = 3 mice/group) (mean ± SEM, 0.73 ± 0.02 for the proaggregation mutant; 0.68 ± 0.07 for the antiaggregation mutant). C, Immunoblots of Sarkosyl-soluble and -insoluble fractions from cortex lysates of proaggregation and antiaggregation mutant mice at 3 and 12 months of gene expression. Blot analysis with K9JA antibody. Sarkosyl-soluble fractions show the presence of endogenous (∼55 kDa) and exogenous (∼12–14 kDa) Tau protein in the case of proaggregation and antiaggregation mutants. The Sarkosyl-insoluble fractions indicate the aggregation of endogenous mouse Tau and exogenous mutant TauRD in the proaggregation mutant mice, but the absence of aggregation in the antiaggregation mutant mice.
Figure 3.
Tau aggregation in TauRD/ΔK280 transgenic mice by Gallyas silver staining. A, Entorhinal cortex of negative control (nontransgenic littermate) at 21 months. B, C, Gallyas-positive neurons in the entorhinal cortex (B) and in amygdala (C) in proaggregation mutant mice at 3 months after TauRD/ΔK280 gene expression. The arrow indicates a ballooned neuron in the amygdala. D, F, Accumulation of Gallyas-positive neurons in the entorhinal cortex at 12 months (D) and in neocortex at 15 months after TauRD/ΔK280 gene expression (F). E, G, Higher magnification of D and F; the arrow in G indicates dystrophic neurites. H, I, Negative stain electron microscopy of bundles of Tau filaments labeled with 10 nm immunogold, using antibodies K9JA (H) (against repeats plus C-terminal domain) and Tau-1 (I) (against region 189–207 aa before the repeats), demonstrating that mouse Tau and exogenous TauRD are present in the same filaments. The filaments are ∼10 nm wide and were isolated from proaggregation transgenic mice at 7 months of gene expression. Scale bars: A–C, E, G, 50 μm; D, F, 100 μm; H, I, 100 nm. Ent, Entorhinal cortex; Amyg, amygdala.
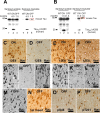
Figure 4.
NFTs in proaggregation but not in antiaggregation mice. A, Gallyas-positive neurons in the entorhinal cortex of proaggregation mutant mice at 3 months after TauRD/ΔK280 gene expression. B, Absence of Gallyas-positive neurons in the entorhinal cortex of antiaggregation mice at 22 months after TauRD/ΔK280/2P gene expression. C, Thioflavin S staining of the entorhinal cortex confirms the presence of NFTs in proaggregation mice at 3 months after TauRD/ΔK280 gene expression and absence of NFTs in the antiaggregation mutant (D) at 3 months after TauRD/ΔK280/2P gene expression. Scale bars: A–D, 20 μm. Ent, Entorhinal cortex.
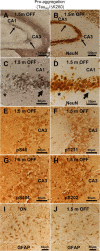
Figure 5.
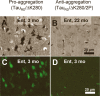
Phosphorylation at KXGS motifs and mislocalization of TauRD/ΔK280 to the somatodendritic compartment. A, Bar diagram of Tau repeat domain and 12E8 epitopes, the phosphorylation sites of MARK. B, Somatic relocalization of hyperphosphorylated TauRD/ΔK280 in hippocampal, CA1 neurons at 2 months after gene expression of the proaggregation mutant. C, Absence of hyperphosphorylated TauRD/ΔK280/2P in hippocampal CA1 region at 2 months after gene expression of the antiaggregation mutant. D, Immunohistochemistry of proaggregation mouse brains at 12 months of TauRD/ΔK280 gene expression showed phosphorylation and translocation of Tau protein in the somatodendritic compartment of subiculum and (E) only mild phosphorylation of Tau protein in the antiaggregation mutant at the same time point. Scale bar: B–E, 20 μm.
Figure 6.
Coaggregation and phosphorylation of endogenous and exogenous Tau in proaggregation mutant mice. A, Coronal section from the brain of proaggregation mice at 5 months of gene expression by Gallyas silver staining. The frames indicate NFTs in the hippocampus and amygdala of proaggregation mutant mice. B, C, Higher magnification of the hippocampus and amygdala. D, E, The same areas as B and C show positive 12E8 staining. F–I, Pyramidal neurons, CA3 region positive for pT231, pS46, pS202, and pS404. These sites lie outside the repeats and demonstrate the coaggregation of mouse Tau. Scale bars: A, 1 mm; B–I, 100 μm. amyg, Amygdala.
Figure 7.
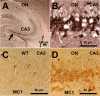
Neurofibrillary pathology and pathological conformation of mouse Tau in the hippocampus of TauRD/ΔK280 proaggregation mice. A, NFT pathology in the case of TauRD/ΔK280 proaggregation mice at 8 months after gene expression. The arrow indicates the CA3 hippocampal neurons stained by Gallyas silver staining. B, Higher magnification of hippocampal CA3 region, indicating formation of NFT pathology in the brain of proaggregation mice. C, D, Conformational changes of endogenous Tau identified by MC1 antibody in the brain of proaggregation mice at 8 months after gene expression (D). The same CA3 region in the wild-type mice is showing the absence of MC1 immunoreactivity (C). Scale bars: A, 100 μm; B–D, 50 μm.
Figure 8.
Neuron loss and astrogliosis in hippocampus of proaggregation transgenic mice. Coronal brain sections were immunostained with NeuN (neuron-specific marker). A–C, Comparison between the layers of granule cells in the dentate gyrus of control, proaggregation, and antiaggregation mice at 5 months of gene expression. Arrows indicate the loss of neurons in the dentate gyrus of the proaggregation mutant. D–F, Control, proaggregation mutant, and antiaggregation mutant at 24 months of gene expression. Note the reduction of the granule cell layer in the dentate gyrus in the case of the proaggregation mutant compared with antiaggregation mutant and control mice. The double arrows indicate the shrinkage of the molecular layer for proaggregation mutant and no changes in the case of antiaggregation mutant and wild-type mice. G–I, Paraffin brain sections immunostained for GFAP. Note increased GFAP immunoreactivity in the hilus of the proaggregation mutant at 21 months of gene expression compared with antiaggregation mutant and wild-type mice, indicating that aggregation of TauRD induces inflammatory reactions. Scale bars: A–F, 100 μm; G–I, 50 μm.
Figure 9.
TauRD/ΔK280 reduces spine-synapse density and synaptic markers. A, Stratum radiatum of CA1 region of hippocampus. Coronal brain sections from control, proaggregation, and antiaggregation mice were immunostained for the presynaptic marker synaptophysin. Immunohistochemistry staining was performed on animals after 9 months of TauRD gene expression and age-matched nontransgenic littermates. B, Quantification of staining shows that synaptophysin is strongly decreased in proaggregation mice [e.g., by ∼70% in the CA1 region (n = 9–16; mean ± SE; p < 0.001) and by ∼56% in the CA3 region (n = 9–16; mean ± SE; * p < 0.05)]. C, Quantification of spinophilin immunostaining in the CA1 and CA3 region shows no significant differences between proaggregation and antiaggregation mice, and a slight decrease compared with nontransgenic littermates (n = 9–16; mean ± SE). D, E, Brain sections from proaggregation and antiaggregation (9.5 months ON) mice were imaged by electron microscopy (stratum radiatum of the CA1 region). F, Quantitative evaluation of spine-synapses in the stratum radiatum of the CA1 hippocampal region. Note the decrease by 27% of the spine synapse number in the proaggregation mutant compared with antiaggregation and control mice (proaggregation mice had 6.7 ± 0.7 spine-synapses per 6.4 μm3; antiaggregation mice had 9.7 ± 0.5 spine synapses per 6.4 μm3; wild-type mice had 9.2 ± 0.6 spine synapses per 6.4 μm3) (n = 10; mean ± SE; *** p < 0.001). Scale bars: A, 40 μm; D, E, 600 nm.
Figure 10.
Phosphorylation and aggregation of Tau is reduced after switching off the expression of TauRD/ΔK280. A, Representative immunoblots of Sarkosyl-soluble and Sarkosyl-insoluble fractions from cortex homogenates of young proaggregation mice: lanes 1–3, Sarkosyl-soluble fractions; and lanes 4–6, Sarkosyl-insoluble fractions of wild-type mice; proaggregation mutant mice 4.5 months ON; or proaggregation mutant mice 3 months ON and 1.5 months OFF. B, Immunoblot analysis of Sarkosyl-soluble fractions (lanes 1–3) and Sarkosyl-insoluble fractions (lanes 4–6) from cortex homogenates in the case of older proaggregation mice. Lanes 1 and 4 show wild type; lanes 2 and 5 show proaggregation mutant, 11.5 months expression ON; and lanes 3 and 6 show proaggregation mutant, 10 months ON and 1.5 months OFF. Note that after switch-off for 1.5 months, the insoluble human TauRD disappears, whereas the insoluble mouse Tau stays aggregated (lane 6 in A and B; red boxes). C, Phosphorylation at KXGS motifs in Tau repeats seen by the 12E8 antibody in the cortex of proaggregation mutant at 4.5 months TauRD/ΔK280 gene expression and (D) loss of 12E8 immunoreactivity after 1.5 months switching off gene expression (3 months ON and 1.5 months OFF). E, F, 12E8 immunoreactivity in proaggregation transgenic mice at 11.5 months gene expression (E) was reversible after suppression of TauRD/ΔK280 gene for 1.5 months (10 months ON and 1.5 months OFF) (F). G, Tangles at 4.5 months of TauRD/ΔK280 gene expression in entorhinal cortex (Gallyas staining). H, I, Two examples of reduction (H) or persistence (I) of tangles in the entorhinal cortex after switching off TauRD/ΔK280 gene expression for 1.5 months in mice with initial 3 months gene expression (3 months ON and 1.5 months OFF). J, NFTs in entorhinal cortex of proaggregation mutant at 11.5 months TauRD/ΔK280 expression. K, L, Two examples of switch off experiments: after 1.5 month suppression of TauRD/ΔK280 gene expression, the tangle pathology decreased (K) or partly persisted (L) in proaggregation mice initially induced for 10 months (10 months ON and 1.5 months OFF). M–P, Entorhinal neurons in the brain of proaggregation mice after 1.5 months of switch off of the TauRD/ΔK280 transgene (10 months ON and 1.5 months OFF) were positive for Gallyas staining (M), and at the same time were stained by antibodies against the first N-terminal insert of Tau with antibody SA4473 (N), against Tau phosphorylated at S46 (O) and at T231 (P). Note that full-length mouse Tau is present in the persisting tangles after switching off exogenous TauRD. Scale bars: C–P, 50 μm.
Figure 11.
Aggregated mouse Tau is toxic to hippocampal neurons. A, NFT pathology (Gallyas silver staining) in the hippocampus of proaggregation mutant mice after switching off the TauRD/ΔK280 transgene expression for 1.5 months (10 months ON and 1.5 months OFF). B, NeuN staining of CA1 and CA3 hippocampal area with NFT pathology displays neuronal loss (arrows) after switching off the transgene for 1.5 months (10 months ON and 1.5 months OFF). C, Higher magnification of A showing NFT pathology by Gallyas silver staining. D, Higher magnification of B showing altered neuronal morphology and loss of neurons in the areas affected by NFT pathology after 1.5 months of switching off the transgene. Note that region of higher silver staining in C corresponds to lower neuron count in D (arrows). In contrast, the areas not affected by NFT pathology do not display altered neuronal morphology (stars). E–H, Phosphorylation of endogenous mouse Tau at S46, T231, S202, and S404 in the CA3 region of proaggregation mutant mice after 1.5 months of switching off gene expression (10 months ON and 1.5 months OFF). Note hyperphosphorylation of aggregated mouse Tau. I, J, The astrocytosis identified by GFAP was reduced in the proaggregation mutant after 1.5 months switching off (J) (4.5 months ON and 1.5 months OFF), compared with the proaggregation mutant with continuous transgene expression (I) (6 months ON). Scale bars: A, B, 100 μm; C–J, 50 μm.
Similar articles
- 'Prion-like' propagation of mouse and human tau aggregates in an inducible mouse model of tauopathy.
Sydow A, Mandelkow EM. Sydow A, et al. Neurodegener Dis. 2010;7(1-3):28-31. doi: 10.1159/000283479. Epub 2010 Feb 13. Neurodegener Dis. 2010. PMID: 20160454 - The beta-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy.
Eckermann K, Mocanu MM, Khlistunova I, Biernat J, Nissen A, Hofmann A, Schönig K, Bujard H, Haemisch A, Mandelkow E, Zhou L, Rune G, Mandelkow EM. Eckermann K, et al. J Biol Chem. 2007 Oct 26;282(43):31755-65. doi: 10.1074/jbc.M705282200. Epub 2007 Aug 23. J Biol Chem. 2007. PMID: 17716969 - Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology.
Hochgräfe K, Sydow A, Mandelkow EM. Hochgräfe K, et al. FEBS J. 2013 Sep;280(18):4371-81. doi: 10.1111/febs.12250. Epub 2013 Apr 22. FEBS J. 2013. PMID: 23517246 Review. - Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau.
Van der Jeugd A, Hochgräfe K, Ahmed T, Decker JM, Sydow A, Hofmann A, Wu D, Messing L, Balschun D, D'Hooge R, Mandelkow EM. Van der Jeugd A, et al. Acta Neuropathol. 2012 Jun;123(6):787-805. doi: 10.1007/s00401-012-0987-3. Epub 2012 Apr 25. Acta Neuropathol. 2012. PMID: 22532069 Free PMC article. - Inhibition of tau aggregation in cell models of tauopathy.
Khlistunova I, Pickhardt M, Biernat J, Wang Y, Mandelkow EM, Mandelkow E. Khlistunova I, et al. Curr Alzheimer Res. 2007 Dec;4(5):544-6. doi: 10.2174/156720507783018307. Curr Alzheimer Res. 2007. PMID: 18220518 Review.
Cited by
- Identification of oligomers at early stages of tau aggregation in Alzheimer's disease.
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR, Kayed R. Lasagna-Reeves CA, et al. FASEB J. 2012 May;26(5):1946-59. doi: 10.1096/fj.11-199851. Epub 2012 Jan 17. FASEB J. 2012. PMID: 22253473 Free PMC article. - Modulation and detection of tau aggregation with small-molecule ligands.
Chang E, Honson NS, Bandyopadhyay B, Funk KE, Jensen JR, Kim S, Naphade S, Kuret J. Chang E, et al. Curr Alzheimer Res. 2009 Oct;6(5):409-14. doi: 10.2174/156720509789207976. Curr Alzheimer Res. 2009. PMID: 19874263 Free PMC article. Review. - Tau local structure shields an amyloid-forming motif and controls aggregation propensity.
Chen D, Drombosky KW, Hou Z, Sari L, Kashmer OM, Ryder BD, Perez VA, Woodard DR, Lin MM, Diamond MI, Joachimiak LA. Chen D, et al. Nat Commun. 2019 Jun 7;10(1):2493. doi: 10.1038/s41467-019-10355-1. Nat Commun. 2019. PMID: 31175300 Free PMC article. - Pathological and physiological functional cross-talks of α-synuclein and tau in the central nervous system.
Jin M, Wang S, Gao X, Zou Z, Hirotsune S, Sun L. Jin M, et al. Neural Regen Res. 2024 Apr;19(4):855-862. doi: 10.4103/1673-5374.382231. Neural Regen Res. 2024. PMID: 37843221 Free PMC article. Review. - AMPA Receptor Trafficking in Natural and Pathological Aging.
Jurado S. Jurado S. Front Mol Neurosci. 2018 Jan 9;10:446. doi: 10.3389/fnmol.2017.00446. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375307 Free PMC article. Review.
References
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. - PMC - PubMed
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
- Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous