2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis - PubMed (original) (raw)
2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis
Christian M Becker et al. Am J Pathol. 2008 Feb.
Abstract
Endometriosis, the presence of ectopic endometrial tissue outside the uterine cavity, is a common disease affecting women during their reproductive years. Current therapeutic success is often unsatisfactory because of limited insight into disease mechanisms. Nevertheless, angiogenesis plays an essential role in the pathogenesis of the disease, making it a potential novel target for therapy. In the current study, we demonstrate in an established mouse model of endometriosis that transient hypoxia in transplanted endometriosis-like lesions results in the up-regulation of hypoxia-inducible factor-1alpha (HIF-1alpha), leading to the expression of vascular endothelial growth factor (VEGF), a key player in endometriosis-associated angiogenesis. Systemic treatment with the angiogenesis inhibitor 2-methoxyestradiol suppressed HIF-1alpha expression in vivo, resulting in a decreased downstream expression of HIF-1alpha target genes, such as for VEGF, phosphoglycerate kinase, and glucose transporter-1. 2-Methoxyestradiol also suppressed VEGF-induced vascular permeability, as demonstrated in a modified Miles assay. Finally, systemic treatment with 2-methoxyestradiol significantly inhibited the growth of endometriosis-like lesions in a dose-dependent manner. In conclusion, hypoxia appears to play an important role in the pathogenesis of endometriosis and endometriosis-associated angiogenesis, and the angiogenesis inhibitor 2-methoxyestradiol may be a potential candidate for systemic treatment in the future.
Figures
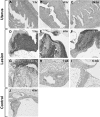
Figure 1
A: Hypoxyprobe staining. Representative murine endometriotic tissue removed at different time points after transplantation. D: 1 hour. E: 4 hours. F: 24 hours. G: 48 hours. H: 1 week. I: 6 months. Each mouse was injected with pimonidazole hydrochloride (hypoxyprobe-1) 30 minutes before removal. Eutopic uterine lesions from the same mice were used as negative controls (A–C). Staining with hypoxyprobe antibody and counterstaining with hematoxylin. Control endometriotic tissue (4 hours; J) stained with an isotype-matched irrelevant murine IgG1 monoclonal antibody directed against Shigela toxin.
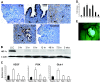
Figure 2
A: Immunohistochemistry staining of endometriosis-like lesions with anti-HIF-1α antibody. Counterstaining with hematoxylin. Increasing immunoreactivity (brown color) in glandular epithelial and stromal cells (arrows) over time (0, 1, 3, and 6 hours). Control (6 hours) stained with nonspecific rabbit IgG antibody. B: Western blot of endometriotic tissue stained with ant-HIF-1α antibody from different time points after transplantation. Loading control of same Western blot stained with anti-β-actin antibody. C: Real-time RT PCR of endometriosis-like lesions at different time points using primers for HIF-1α-dependent genes (VEGF, PGK, and Glut-1). Bars indicate SEM. D: Semiquantitative measurement of Western blot for HIF-1α as seen in B. Error bars indicate SEM. E: Endometriosis-like lesion from a green fluorescent protein+/+ mouse on the peritoneal wall of a wild-type recipient. Typical growth of blood vessels into the endometriosis-like lesion.
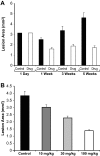
Figure 3
Systemic 2-methoxyestradiol inhibits growth of endometriosis-like lesions. Endometriosis-like lesions were measured in two perpendicular diameters (_D_1 and _D_2) with a caliper, and CSA was calculated using the formula of an ellipse (_D_1 × _D_2 × π/4) as previously described.,, A: Mean CSA of endometriosis-like lesions (n = 7 lesions) of mice treated with vehicle (▪) or 2-methoxyestradiol (□) (n = 6/group). B: Mean CSA after 4 weeks of oral treatment with different doses of 2-methoxyestradiol. Bars indicate the SEM.
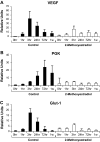
Figure 4
Systemic 2-methoxyestradiol treatment reduces expression of HIF-1α target genes. Results of a representative experiment using real-time RT-PCR of endometriosis-like lesions removed after different time intervals after autotransplantation. Mice had been treated systemically with 2-methoxyestradiol before the induction of endometriosis. Lesions were removed at different time points (baseline, 1 hour, 3 hours, 24 hours, 72 hours, and 1 week) and tested for mRNA expression of VEGF (A), PGK (B), and Glut-1 (C). Bars indicate SEM.
Figure 5
A: Western blot of endometriotic tissue samples at baseline (0 hours) and 6 hours. Lewis lung carcinoma is used as positive control in equivalent concentration. Staining with anti-HIF-1α antibody and anti-β actin as loading control. B: A modified Miles assay shows that systemic 2-methoxyestradiol treatment inhibits vascular permeability. Mice were pretreated orally with tap water (control), vehicle, and 2-methoxyestradiol for 5 days and then anesthetized and injected with Evans blue (i.v.). After 10 minutes, mice were given intradermal injections of PBS (50 μl; top two spots) and VEGF/vascular permeability factor (50 ng in 50 μl; bottom two spots), and mice were euthanized after 20 minutes. Reduced extravasation of dye in the skin of mice treated with 2-methoxyestradiol. C: Dye from excised skin was extracted with formamide for 5 days, and dye contents were measured at 620 nm. Data are expressed as mean ± SEM.
Similar articles
- 2-methoxyestradiol in the pathophysiology of endometriosis: focus on angiogenesis and therapeutic potential.
Machado-Linde F, Pelegrin P, Sanchez-Ferrer ML, Leon J, Cascales P, Parrilla JJ. Machado-Linde F, et al. Reprod Sci. 2012 Oct;19(10):1018-29. doi: 10.1177/1933719112446080. Epub 2012 Aug 8. Reprod Sci. 2012. PMID: 22875846 Review. - Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway.
Park JJ, Hwang SJ, Park JH, Lee HJ. Park JJ, et al. Cell Oncol (Dordr). 2015 Apr;38(2):111-8. doi: 10.1007/s13402-014-0216-2. Epub 2015 Jan 6. Cell Oncol (Dordr). 2015. PMID: 25561311 - Effect of connective tissue growth factor on hypoxia-inducible factor 1alpha degradation and tumor angiogenesis.
Chang CC, Lin MT, Lin BR, Jeng YM, Chen ST, Chu CY, Chen RJ, Chang KJ, Yang PC, Kuo ML. Chang CC, et al. J Natl Cancer Inst. 2006 Jul 19;98(14):984-95. doi: 10.1093/jnci/djj242. J Natl Cancer Inst. 2006. PMID: 16849681 - Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints.
Gelse K, Pfander D, Obier S, Knaup KX, Wiesener M, Hennig FF, Swoboda B. Gelse K, et al. Arthritis Res Ther. 2008;10(5):R111. doi: 10.1186/ar2508. Epub 2008 Sep 12. Arthritis Res Ther. 2008. PMID: 18789153 Free PMC article. - Mechanistic and therapeutic implications of angiogenesis in endometriosis.
Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, Sidell N. Taylor RN, et al. Reprod Sci. 2009 Feb;16(2):140-6. doi: 10.1177/1933719108324893. Epub 2008 Nov 11. Reprod Sci. 2009. PMID: 19001553 Free PMC article. Review.
Cited by
- Indole-3-Carbinol Inhibits the Growth of Endometriotic Lesions by Suppression of Microvascular Network Formation.
Rudzitis-Auth J, Becker M, Scheuer C, Menger MD, Laschke MW. Rudzitis-Auth J, et al. Nutrients. 2022 Nov 21;14(22):4940. doi: 10.3390/nu14224940. Nutrients. 2022. PMID: 36432626 Free PMC article. - Lipoxin A₄ prevents the progression of de novo and established endometriosis in a mouse model by attenuating prostaglandin E₂ production and estrogen signaling.
Kumar R, Clerc AC, Gori I, Russell R, Pellegrini C, Govender L, Wyss JC, Golshayan D, Canny GO. Kumar R, et al. PLoS One. 2014 Feb 24;9(2):e89742. doi: 10.1371/journal.pone.0089742. eCollection 2014. PLoS One. 2014. PMID: 24587003 Free PMC article. - Vascularisation in Deep Endometriosis: A Systematic Review with Narrative Outcomes.
Powell SG, Sharma P, Masterson S, Wyatt J, Arshad I, Ahmed S, Lash G, Cross M, Hapangama DK. Powell SG, et al. Cells. 2023 May 5;12(9):1318. doi: 10.3390/cells12091318. Cells. 2023. PMID: 37174718 Free PMC article. Review. - Endometriosis, a disease of the macrophage.
Capobianco A, Rovere-Querini P. Capobianco A, et al. Front Immunol. 2013 Jan 28;4:9. doi: 10.3389/fimmu.2013.00009. eCollection 2013. Front Immunol. 2013. PMID: 23372570 Free PMC article. - A phase I dose-escalation, safety and pharmacokinetic study of the 2-methoxyestradiol analog ENMD-1198 administered orally to patients with advanced cancer.
Zhou Q, Gustafson D, Nallapareddy S, Diab S, Leong S, Lewis K, Gore L, Messersmith WA, Treston AM, Eckhardt SG, Sidor C, Camidge DR. Zhou Q, et al. Invest New Drugs. 2011 Apr;29(2):340-6. doi: 10.1007/s10637-009-9383-9. Epub 2010 Jan 19. Invest New Drugs. 2011. PMID: 20084425 Free PMC article. Clinical Trial.
References
- ASRM: Endometriosis and infertility. Fertil Steril. 2004;81:1441–1446. - PubMed
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endothelial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469.
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. discussion 118, 396–406. - PubMed
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases