Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin - PubMed (original) (raw)
Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin
Antonina Roll-Mecak et al. Nature. 2008.
Abstract
Spastin, the most common locus for mutations in hereditary spastic paraplegias, and katanin are related microtubule-severing AAA ATPases involved in constructing neuronal and non-centrosomal microtubule arrays and in segregating chromosomes. The mechanism by which spastin and katanin break and destabilize microtubules is unknown, in part owing to the lack of structural information on these enzymes. Here we report the X-ray crystal structure of the Drosophila spastin AAA domain and provide a model for the active spastin hexamer generated using small-angle X-ray scattering combined with atomic docking. The spastin hexamer forms a ring with a prominent central pore and six radiating arms that may dock onto the microtubule. Helices unique to the microtubule-severing AAA ATPases surround the entrances to the pore on either side of the ring, and three highly conserved loops line the pore lumen. Mutagenesis reveals essential roles for these structural elements in the severing reaction. Peptide and antibody inhibition experiments further show that spastin may dismantle microtubules by recognizing specific features in the carboxy-terminal tail of tubulin. Collectively, our data support a model in which spastin pulls the C terminus of tubulin through its central pore, generating a mechanical force that destabilizes tubulin-tubulin interactions within the microtubule lattice. Our work also provides insights into the structural defects in spastin that arise from mutations identified in hereditary spastic paraplegia patients.
Figures
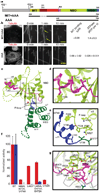
Figure 1. X-ray structure of the nucleotide-free AAA domain of spastin
a, Domain structure of Drosophila spastin: grey, N-terminal domain; red, linker (exon 4, absent in the shorter isoform of spastin used in this study, is hatched); and the AAA domain (coloured according to the X-ray structure). NBD, nucleotide-binding domain; HBD, four-helix bundle domain. Two potential start codons (ATG) are shown (see Supplementary Methods for discussion). The N-terminal boundary of the AAA domain is based on our X-ray structure and differs from that of ref. . A segment of the structurally important N-terminal helix of the AAA domain is within what the authors of ref. define as a microtubule-binding domain. The MIT + AAA and AAA constructs are shown schematically below. b, Left, MIT + AAA disassembles the microtubule network when transfected in Drosophila S2 cells and when added to microtubules in vitro, but AAA has no detectable activity at the same concentration (0.15 µM). (Weak severing is observed at higher concentrations, Supplementary Fig. 1.) Arrows indicate breaks in microtubules. Scale bar, 5 µm. Right, microtubule (MT)-binding and ATPase activities of MIT + AAA and AAA. Microtubule-binding affinity was determined for the Walker B E583Q mutant, which is a stable hexamer and is inactive in severing. c, Ribbon representation of the spastin AAA domain crystal structure. N-terminal helix/loop, magenta; NBD, light green; HBD, dark green; C-terminal helix, blue. The pink sphere depicts a chloride ion. d, Conserved hydrophobic interactions between the N-terminal helix and the main body of the NBD. e, Conserved interactions between the C-terminal helix and the P loop. f, ATPase (red) and microtubule-severing (blue) rates of N- and C-terminal helix mutants. Error bars represent standard errors of the mean (see Methods). WT, wild type. g, Detail of the superposition of spastin and ATP-bound NSF structures, showing contacts that keep the N-terminal flap of monomeric spastin (magenta) in an open conformation, unable to stabilize the nucleotide or interact with the neighbouring protomer. Spastin is colour-coded as in panel c. NSF is in grey. Dashed lines, hydrogen bonds.
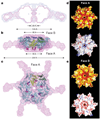
Figure 2. Model of active, hexameric spastin from light and small-angle X-ray scattering
a, Ab initio SAXS reconstructions of C. elegans spastin (MIT + AAA; residues 15–452, ATP-hydrolysis-deficient E278Q mutant). Shown is a cross-section through the filtered (magenta) and composite (blue) SAXS envelopes. The composite structure consists of the aligned, superimposed and summed models from seven independent simulations, whereas the filtered model corresponds to the most probable density map. b, c, Fit of a spastin hexameric model into the SAXS reconstruction (equatorial (b) and axial (c) views). In the absence of an atomic model of the MIT + linker, its precise location within the envelope is uncertain. Maximal diameter is given at various heights of the structure. c shows face A of the hexamer. For details, see Methods. Colour-coding for spastin is as in Fig. 1c. d, e, Surface properties of face A (d) and face B (e). Top image of d and e, solvent-accessible surface of the spastin hexamer model, colour-coded for amino acid similarity as in Supplementary Fig. 3 (white, 40% identity, to dark red, 100% identity, among spastin and katanins). Bottom, solvent-accessible surface of the spastin hexamer model, colour-coded for electrostatic potential (red, negative; blue, positive, ranging from −12 kT to 12 kT).
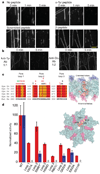
Figure 3. Role of the tubulin C terminus and the spastin pore in microtubule-severing
a, Effects of tubulin C-terminal peptides on microtubule-severing in vitro. Addition of α-Tyr peptide had no detectable effect on severing rates, whereas a β-tubulin C-terminal peptide reduced the severing rate. A scrambled peptide had no detectable effect (see Methods). Scale bar, 5 µm. b, Antibodies (Ab) recognizing Glu–α-tubulin, β-tubulin and polyglutamylated tubulin (anti-‘Glu’) inhibit spastin-mediated severing completely at 1:2 antibody:tubulin molar ratio (the same level of protection was seen even 30 min after spastin addition), whereas antibodies recognizing Tyr– α-tubulin (anti-Tyr) did not protect against severing, even at a 5:1 antibody:tubulin molar ratio (antibody binding to these microtubules demonstrated in Supplementary Fig. 7b). c, Left, conservation of the three pore loops. Loop 1 residues are conserved in all AAA ATPases; loops 2 and 3 are specific to the spastin subfamily. Effects on microtubule-severing of mutations in pore loop residues are shown on top of the alignment: red, inactive; black, severely crippled; green, active. Asterisks denote disease mutations. The effects of mutations generally decrease in severity from the pore entrance to the exit, with loop 1 being the least permissive to substitutions. Right, positions of pore loops (labelled 1, 2 and 3) in the spastin hexamer, in a cross-sectional view of the pore. Side-chains for residues K555, Y556 and D559 as well as residues 592–596 are not visible in the electron density maps and are presumed to be disordered. d, Left, ATPase (red) and microtubule-severing (blue) rates for selected mutants. Error bars represent standard errors of the mean (see Methods). Right, molecular surface of the hexameric spastin model showing in yellow the location of residues on face A that impair severing. Loop residues that impair severing are shown in red.
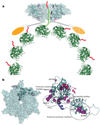
Figure 4. Proposed mechanism of severing by spastin and effects of disease mutations
a, Proposed mechanism for microtubule-severing by spastin. The spastin AAA core is shown in cyan with pore loops 1, 2 and 3 highlighted in red and numbered in the figure. The MIT domains are shown as gold ovals. The valency of the interaction of the MIT domains with the microtubule is unknown. On the basis of affinity measurements, it is likely that not all MIT domains are engaged with the microtubule (the potentially unengaged MIT domain is shown hatched). The tubulin heterodimers forming the microtubule are shown in green as a ribbon representation, whereas the C-terminal tubulin tails are shown in red cartoon representation. b, Left, molecular surface of spastin (face A). One protomer is shown in a ribbon representation and residues mutated in HSP patients are shown as violet spheres. Right, in addition to mapping to the pore loops (S589Y, R601L, P631L), disease mutations can interfere with ATP binding (F522C, N527K, K529R) and protomer–protomer interactions (D697N, R704Q, R641C, R601L, P631L). G511R maps to a loop on face A where it could destabilize protomer–protomer interactions and/or the microtubule-binding interface (Supplementary Fig. 4).
Similar articles
- An allosteric network in spastin couples multiple activities required for microtubule severing.
Sandate CR, Szyk A, Zehr EA, Lander GC, Roll-Mecak A. Sandate CR, et al. Nat Struct Mol Biol. 2019 Aug;26(8):671-678. doi: 10.1038/s41594-019-0257-3. Epub 2019 Jul 8. Nat Struct Mol Biol. 2019. PMID: 31285604 Free PMC article. - Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing.
White SR, Evans KJ, Lary J, Cole JL, Lauring B. White SR, et al. J Cell Biol. 2007 Mar 26;176(7):995-1005. doi: 10.1083/jcb.200610072. J Cell Biol. 2007. PMID: 17389232 Free PMC article. - Conserved aromatic and basic amino acid residues in the pore region of Caenorhabditis elegans spastin play critical roles in microtubule severing.
Matsushita-Ishiodori Y, Yamanaka K, Hashimoto H, Esaki M, Ogura T. Matsushita-Ishiodori Y, et al. Genes Cells. 2009 Aug;14(8):925-40. doi: 10.1111/j.1365-2443.2009.01320.x. Epub 2009 Jul 13. Genes Cells. 2009. PMID: 19619244 - The AAA ATPase spastin links microtubule severing to membrane modelling.
Lumb JH, Connell JW, Allison R, Reid E. Lumb JH, et al. Biochim Biophys Acta. 2012 Jan;1823(1):192-7. doi: 10.1016/j.bbamcr.2011.08.010. Epub 2011 Aug 25. Biochim Biophys Acta. 2012. PMID: 21888932 Review. - Hereditary spastic paraplegia SPG4: what is known and not known about the disease.
Solowska JM, Baas PW. Solowska JM, et al. Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review.
Cited by
- Transcriptional Profiles of Skeletal Muscle Associated With Increasing Severity of White Striping in Commercial Broilers.
Malila Y, Uengwetwanit T, Arayamethakorn S, Srimarut Y, Thanatsang KV, Soglia F, Strasburg GM, Rungrassamee W, Visessanguan W. Malila Y, et al. Front Physiol. 2020 Jun 16;11:580. doi: 10.3389/fphys.2020.00580. eCollection 2020. Front Physiol. 2020. PMID: 32612536 Free PMC article. - Binding of Substrates to the Central Pore of the Vps4 ATPase Is Autoinhibited by the Microtubule Interacting and Trafficking (MIT) Domain and Activated by MIT Interacting Motifs (MIMs).
Han H, Monroe N, Votteler J, Shakya B, Sundquist WI, Hill CP. Han H, et al. J Biol Chem. 2015 May 22;290(21):13490-9. doi: 10.1074/jbc.M115.642355. Epub 2015 Apr 1. J Biol Chem. 2015. PMID: 25833946 Free PMC article. - Multiparametric rapid screening of neuronal process pathology for drug target identification in HSP patient-specific neurons.
Rehbach K, Kesavan J, Hauser S, Ritzenhofen S, Jungverdorben J, Schüle R, Schöls L, Peitz M, Brüstle O. Rehbach K, et al. Sci Rep. 2019 Jul 3;9(1):9615. doi: 10.1038/s41598-019-45246-4. Sci Rep. 2019. PMID: 31270336 Free PMC article. - Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration.
Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Rostovtseva TK, et al. Proc Natl Acad Sci U S A. 2008 Dec 2;105(48):18746-51. doi: 10.1073/pnas.0806303105. Epub 2008 Nov 24. Proc Natl Acad Sci U S A. 2008. PMID: 19033201 Free PMC article. - The ESCRT machinery: from the plasma membrane to endosomes and back again.
Schuh AL, Audhya A. Schuh AL, et al. Crit Rev Biochem Mol Biol. 2014 May-Jun;49(3):242-61. doi: 10.3109/10409238.2014.881777. Epub 2014 Jan 24. Crit Rev Biochem Mol Biol. 2014. PMID: 24456136 Free PMC article. Review.
References
- Hazan J, et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nature Genet. 1999;23:296–303. - PubMed
- Frickey T, Lupas AN. Phylogenetic analysis of AAA proteins. J. Struct. Biol. 2004;146:2–10. - PubMed
- Roll-Mecak A, Vale RD. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr. Biol. 2005;15:650–655. - PubMed
- Salinas S, et al. Human spastin has multiple microtubule-related functions. J. Neurochem. 2005;95:1411–1420. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases