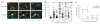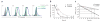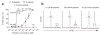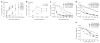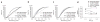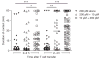T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation - PubMed (original) (raw)
doi: 10.1038/ni1559. Epub 2008 Jan 20.
Thorsten R Mempel, Irina B Mazo, Bai Liu, Maxim N Artyomov, Huan Zheng, Antonio Peixoto, Michael P Flynn, Balimkiz Senman, Tobias Junt, Hing C Wong, Arup K Chakraborty, Ulrich H von Andrian
Affiliations
- PMID: 18204450
- PMCID: PMC2698867
- DOI: 10.1038/ni1559
T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation
Sarah E Henrickson et al. Nat Immunol. 2008 Mar.
Abstract
After homing to lymph nodes, CD8+ T cells are primed by dendritic cells (DCs) in three phases. During phase one, T cells undergo brief serial contacts with DCs for several hours, whereas phase two is characterized by stable T cell-DC interactions. We show here that the duration of phase one and T cell activation kinetics correlated inversely with the number of complexes of cognate peptide and major histocompatibility complex (pMHC) per DC and with the density of antigen-presenting DCs per lymph node. Very few pMHC complexes were necessary for the induction of full-fledged T cell activation and effector differentiation. However, neither T cell activation nor transition to phase two occurred below a threshold antigen dose determined in part by pMHC stability. Thus, phase one permits T cells to make integrated 'measurements' of antigen dose that determine subsequent T cell participation in immune responses.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureimmunology/.
Figures
Figure 1
Interactions of P14 T cells with DCs pulsed with C-peptide or M-peptide in vivo. (a) MP-IVM of P14 T cells (green) and OT-I control T cells (blue) interacting with DCs (red) pulsed with 10 μM C-peptide (top) or 10 μM M-peptide (bottom). Still images were obtained from individual time-lapse movies at 2.5 h after T cell injection; images were chosen in 75-second intervals from movies with cycle times of 15 s. Green and blue arrows indicate P14 T cells and control T cells, respectively, interacting with DCs; n = 3–4 mice for each condition. Original magnification, × 60. (b) Duration of P14 T cell contacts with DCs pulsed with 10 μM M-peptide (M) or C-peptide (C). Data are from 60-minute recordings obtained during various time intervals after T cell injection. Data are pooled from two to four mice per time point; small horizontal lines indicate the median. Contact durations are significantly longer with M-peptide than with C-peptide for all time intervals (P < 0.0001; Mann-Whitney U-test). (c) Motility coefficients for P14 T cells interacting with DCs pulsed with 10 μM C-peptide or M-peptide. *, P < 0.05 (Mann-Whitney U-test); n = 2–4 mice per time point (error bars, mean ± s.e.m). Data are representative of two to three (a) or three to five (b, c) experiments.
Figure 2
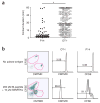
Number of cognate pMHC complexes on DCs. (a) Flow cytometry of the binding of P14 TCR tetramers to 5T33 cells (APCs) pulsed for 3 h at 37 °C with 10 μM C-peptide, M-peptide or control peptide (SIINFEKL) in the presence of peptide-loading enhancer; after cells were washed and incubated at 37 °C (time, above plots) to allow peptide dissociation from H-2Db, cognate pMHC complexes were counted by staining of cells with phycoerythrin-labeled P14 TCR tetramers and annexin V. Histograms are gated on annexin V–negative cells. Data are representative of three experiments. (b) Staining intensity of P14 TCR tetramers bound to 5T33 cells pulsed with C-peptide or M-peptide. The MFI of delayed staining is presented relative to the MFI of cells stained immediately after peptide pulsing. Data represent the mean ± s.e.m of results pooled from three independent experiments. (c) Cognate pMHC complexes remaining after pulsing, calculated on the basis of a measured starting value of 2.5 × 104 H-2Db complexes per DC and calculated half-lives of 6.01 h and 2.36 h for M-peptide and C-peptide, respectively. Lines connecting data lines to axes indicate estimated number of cognate pMHC complexes per DC at the time of T cell injection (18 h after DC pulsing; dashed lines) and 6 h after T cell injection (24 h after DC pulsing; solid lines).
Figure 3
Peptide dose dependence of T cell activation. (a) T cell proliferation after CD11c+ DCs pulsed with M-peptide or C-peptide (concentration, horizontal axis, and as labeled for M-peptide) or with 10 μM control peptide (SIINFEKL) were injected into footpads of CD45.1+ recipient mice, then CFSE-labeled CD45.2+ P14 T cells were injected 18 h later; after 48 h, CFSE dilution was measured in single-cell suspensions of popliteal lymph nodes. *, P < 0.0005; **, P < 0.0001; ***, P < 0.016 (two-tailed Student’s _t_-test). Data (mean ± s.d.) are pooled from three independent experiments with two to six mice per concentration. (b) Cytotoxicity of P14 T cells stimulated in vivo. Purified DCs were pulsed with 10 μM or 200 pM M-peptide or 10 μM control peptide and injected into the footpads of recipient mice; 5 × 106 P14 T cells were injected intravenously 18 h later and, after an additional 48 h, two polyclonal B cell populations (one pulsed with 10 μM M-peptide and labeled with 2 μM CFSE; the other labeled with 0.1 μM CFSE), mixed at a ratio of 1:1, were injected intravenously; 6 h later, the ratio of CFSEhi B cells to CFSElo B cells was assessed in the popliteal lymph node. Numbers above bracketed lines indicate percent CFSEhi B cells (right) or CFSElo B cells (left). Data are pooled from two independent experiments with four to five mice per group.
Figure 4
Dose-dependent effects of C-peptide and M-peptide on T cell–DC interaction dynamics in vivo. MP-IVM analysis of interactions of fluorescent P14 T cells with DCs pulsed with various concentrations of C-peptide or M-peptide in popliteal lymph nodes at various times after T cell injection; durations of individual T cell–DC contacts were analyzed in reconstructed three-dimensional movies. (a) Effect of the pulsing concentration of M-peptide on T cell–DC contact duration; the median duration of all contacts in individual 60-minute recordings was determined for one to four mice per time point. Data are the mean ± s.e.m. of 19 experiments. (b) Effect of the pulsing concentration of C-peptide on T cell–DC contact durations. Data are the mean ± s.e.m. of six experiments with one to three mice per time point per dose. Gray horizontal lines (a, b) separate phase-one interactions (<30 min) from phase-two interactions (>30 min). (c–e) Cumulative dissociation curves of P14 T cells after contact with DCs pulsed with various concentrations of M-peptide or C-peptide, for recordings obtained 2–4 h (c), 4–6 h (d) or 6–8 h (e) after T cell injection. Data are representative of 10–11 experiments with two to three (c, e) or two to four (d) mice per dose.
Figure 5
Effect of antigen dose on the meandering of T cells in lymph nodes. MP-IVM analysis of interactions between fluorescent P14 T cells and DCs pulsed with various concentrations of C-peptide or M-peptide, assessed in popliteal lymph nodes at various times after T cell injection; cell centroids in three dimensions were measured by semiautomated cell tracking and the ‘meandering index’ was calculated by division of the displacement for each cell track by the total path length for that cell track. (a–c) Cumulative distribution plots of the ‘meandering index’ of P14 T cells interacting with DCs pulsed with one of four peptide doses (key) at 2–4 h (a), 4–6 h (b) or 6–8 h (c) after T cell injection. Data are representative of 00 experiments with two to three (a, c) or two to four (b) mice per dose. (d) Median ‘meandering index’ of P14 T cells interacting with DCs pulsed with one of four peptide doses (key) and of OT-I T cells (pooled interactions with DCs pulsed with 100 pM M-peptide, 200 pM M-peptide, 10 μM M-peptide, 10 μM C-peptide or 100 μM C-peptide), assessed at various time intervals (horizontal axis). Data are representative of 10–12 experiments with two to four mice per dose per time point for P14 T cells and 21 experiments with eleven to fourteen mice per time point for OT-I T cells.
Figure 6
Tissue concentration of cognate antigen–bearing DCs controls T cell proliferation and IFN-γ production. CFSE dilution and IFN-γ production of cells in popliteal lymph nodes after subcutaneous injection of a constant number of DCs (5 × 105) and 10 ng LPS into footpads of congenic (CD45.1+) recipients; the fraction of antigen-bearing DCs was varied from 0% to 100% (above plots, a; horizontal axes, b, c) by mixture of DCs pulsed with M-peptide (10 μM or 200 pM) and DCs pulsed with 10 μM control peptide (SIINFEKL). Each recipient received a positive control injection of 100% antigen-bearing DCs in the left footpad and 10%, 1%, 0.1% or 0% antigen-bearing DCs in the right footpad. After 18 h, CFSE-labeled P14 T cells were injected intravenously, followed 2 h later by anti-L-selectin and analysis by flow cytometry 18 h and 48 h later. (a) Flow cytometry of CFSE dilution versus IFN-γ production. (b) Percent proliferated T cells, calculated as a ratio for each mouse by division of percent proliferation in the test popliteal lymph node by that in the positive control popliteal lymph node, which received 100% DCs pulsed with M-peptide. (c) MFI of IFN-γ in transferred P14 T cells in the test popliteal lymph node, presented as a percentage of the MFI for transferred P14 T cells in the control popliteal lymph node, which received 100% DCs pulsed with M-peptide. Data are pooled from three independent experiments with two to five mice per condition per time point (mean ± s.e.m., b, c).
Figure 7
Early phase two for antigen-specific T cells interacting with threshold dose antigen-pulsed DCs when a second DC population pulsed with high-dose antigen is also in the lymph node. MP-IVM analysis of P14 T cell interactions with DCs in popliteal lymph nodes versus a single population or two populations of differentially peptide-pulsed DCs, assessed at various times after T cell injection. P14 T cells interacted with DCs pulsed with 200 pM M-peptide alone (200 pM alone), with DCs pulsed with 200 pM M-peptide in the presence of DCs pulsed with 10 μM M-peptide (200 pM plus 10 μM) or with DCs pulsed with 10 μM M-peptide in the presence of DCs pulsed with 200 pM M-peptide (10 μM plus 200 pM). The duration of individual T cell–DC contacts was analyzed in reconstructed three-dimensional movies; short horizontal lines indicate the median. For 0–2 h, interactions are significantly longer with DCs pulsed with 10 μM M-peptide than with either population of DCs pulsed with 200 pM M-peptide (***, P < 0.0001 for 200 pM alone; *, P = 0.0031 for 200 pM plus 10 μM; Mann-Whitney U-test); for 2–4 h, interactions are significantly shorter for DCs pulsed with 200 pM M-peptide than for DCs pulsed in either combination (**, P = 0.0002 for 200 pM plus 10 μM; ***, P < 0.0001 for 10 μM plus 200 pM; Mann-Whitney U-test). Data are pooled from four experiments with four mice per time point.
Figure 8
DCs can simultaneously maintain phase one– and phase two–like interactions with two different T cell populations. (a) MP-IVM analysis of T cell–DC interactions in popliteal lymph nodes for DCs pulsed with both 10 μM SIINFEKL peptide and 200 pM M-peptide and injected into footpads; after 18 h, P14 and OT-I T cells labeled with different fluorescent dyes were injected intravenously. Duration of individual contacts was analyzed in three-dimensional time-lapse movies. *, P < 0.0001 (Mann-Whitney U-test). Data are representative of two experiments with results pooled from four 1-hour recordings from 1.5 h to 5 h after T cell transfer. (b) Flow cytometry of the proliferation of P14 and OT-I T cells among CD45.2+ cells obtained from the popliteal lymph nodes of CD45.1+ mice injected with DCs left unpulsed (left footpad) or pulsed with 10 μM SIINFEKL and 200 pM M-peptide (right footpad) and, after 18 h, injected intravenously with CFSE-labeled P14 T cells and CMTMR-labeled OT-I T cells (5 × 106 of each), followed by analysis after an additional 48 h. Numbers above bracketed lines indicate percent cells that had divided at least once. Data are representative of two experiments with three mice.
Comment in
- How T cells 'find' the right dendritic cell.
Shaw AS. Shaw AS. Nat Immunol. 2008 Mar;9(3):229-30. doi: 10.1038/ni0308-229. Nat Immunol. 2008. PMID: 18285771 No abstract available.
Similar articles
- In vivo imaging of T cell priming.
Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn M, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. Henrickson SE, et al. Sci Signal. 2008 Mar 25;1(12):pt2. doi: 10.1126/stke.112pt2. Sci Signal. 2008. PMID: 18364513 - Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes.
Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Skokos D, et al. Nat Immunol. 2007 Aug;8(8):835-44. doi: 10.1038/ni1490. Epub 2007 Jul 15. Nat Immunol. 2007. PMID: 17632517 - Antigen availability determines CD8⁺ T cell-dendritic cell interaction kinetics and memory fate decisions.
Henrickson SE, Perro M, Loughhead SM, Senman B, Stutte S, Quigley M, Alexe G, Iannacone M, Flynn MP, Omid S, Jesneck JL, Imam S, Mempel TR, Mazo IB, Haining WN, von Andrian UH. Henrickson SE, et al. Immunity. 2013 Sep 19;39(3):496-507. doi: 10.1016/j.immuni.2013.08.034. Immunity. 2013. PMID: 24054328 Free PMC article. - Antigen-presentation properties of plasmacytoid dendritic cells.
Villadangos JA, Young L. Villadangos JA, et al. Immunity. 2008 Sep 19;29(3):352-61. doi: 10.1016/j.immuni.2008.09.002. Immunity. 2008. PMID: 18799143 Review. - Modulation of T cell function by TCR/pMHC binding kinetics.
Carreño LJ, González PA, Kalergis AM. Carreño LJ, et al. Immunobiology. 2006;211(1-2):47-64. doi: 10.1016/j.imbio.2005.09.003. Epub 2006 Jan 4. Immunobiology. 2006. PMID: 16446170 Review.
Cited by
- Modulation of dendritic cell function by the radiation-mediated secretory protein γ-synuclein.
Kang SM, Kim MH, Song KH, Jung SY, Ahn J, Hwang SG, Lee JH, Lim DS, Song JY. Kang SM, et al. Cell Death Discov. 2015 Jul 27;1:15011. doi: 10.1038/cddiscovery.2015.11. eCollection 2015. Cell Death Discov. 2015. PMID: 27551446 Free PMC article. - The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude.
Jenkins MK, Moon JJ. Jenkins MK, et al. J Immunol. 2012 May 1;188(9):4135-40. doi: 10.4049/jimmunol.1102661. J Immunol. 2012. PMID: 22517866 Free PMC article. Review. - In vivo two-photon imaging of T cell motility in joint-draining lymph nodes in a mouse model of rheumatoid arthritis.
Kobezda T, Ghassemi-Nejad S, Glant TT, Mikecz K. Kobezda T, et al. Cell Immunol. 2012 Jul-Aug;278(1-2):158-65. doi: 10.1016/j.cellimm.2012.08.003. Epub 2012 Sep 5. Cell Immunol. 2012. PMID: 23023071 Free PMC article. - Dendritic cells enhance the antigen sensitivity of T cells.
Garbi N, Kreutzberg T. Garbi N, et al. Front Immunol. 2012 Dec 26;3:389. doi: 10.3389/fimmu.2012.00389. eCollection 2012. Front Immunol. 2012. PMID: 23272004 Free PMC article. - Understanding the structure and function of the immunological synapse.
Dustin ML, Chakraborty AK, Shaw AS. Dustin ML, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a002311. doi: 10.1101/cshperspect.a002311. Epub 2010 Sep 15. Cold Spring Harb Perspect Biol. 2010. PMID: 20843980 Free PMC article. Review.
References
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. - PubMed
- Casrouge A, et al. Size estimate of the αβ TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. - PubMed
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
- Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL066987/HL/NHLBI NIH HHS/United States
- P01 CA071932/CA/NCI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- P01 AI071195-01/AI/NIAID NIH HHS/United States
- AI069259/AI/NIAID NIH HHS/United States
- P01 AI071195/AI/NIAID NIH HHS/United States
- R01 AI072252/AI/NIAID NIH HHS/United States
- R01 AI069259/AI/NIAID NIH HHS/United States
- P01 CA071932-11A2/CA/NCI NIH HHS/United States
- HL066987/HL/NHLBI NIH HHS/United States
- T32 HL007623/HL/NHLBI NIH HHS/United States
- AI072252/AI/NIAID NIH HHS/United States
- HL07623/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials