Adenosine A2A receptor signaling regulation of cardiac NADPH oxidase activity - PubMed (original) (raw)
Adenosine A2A receptor signaling regulation of cardiac NADPH oxidase activity
David Ribé et al. Free Radic Biol Med. 2008.
Abstract
Cardiac tissues express constitutively an NADPH oxidase, which generates reactive oxygen species (ROS) and is involved in redox signaling. Myocardial metabolism generates abundant adenosine, which binds to its receptors and plays important roles in cardiac function. The adenosine A2A receptor (A2AR) has been found to be expressed in cardiac myocytes and coronary endothelial cells. However, the role of the A2AR in the regulation of cardiac ROS production remains unknown. We found that knockout of A2AR significantly decreased (39+/-8%) NADPH-dependent O2- production in mouse hearts compared to age (10 weeks)-matched wild-type controls. This was accompanied by a significant decrease in Nox2 (a catalytic subunit of NADPH oxidase) protein expression, and down-regulation of ERK1/2, p38MAPK, and JNK phosphorylation (all P<0.05). In wild-type mice, intraperitoneal injection of the selective A2AR antagonist SCH58261 (3-10 mg/kg body weight for 90 min) inhibited phosphorylation of p47phox (a regulatory subunit of Nox2), which was accompanied by a down-regulated cardiac ROS production (48+/-8%), and decreased JNK and ERK1/2 activation by 54+/-28% (all P<0.05). In conclusion, A2AR through MAPK signaling regulates p47phox phosphorylation and cardiac ROS production by NADPH oxidase. Modulation of A2AR activity may have potential therapeutic applications in controlling ROS production by NADPH oxidase in the heart.
Figures
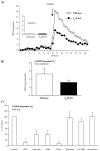
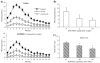
Fig. 1
O2•− production in wild-type and A2AR knockout heart homogenates detected by lucigenin chemiluminescence. (A) Kinetic measurement of O2•− at every minute for a total of 30 min. NADPH was added after 15 min of measurement. (B) Difference in NADPH-dependent O2•− production between wild-type and A2AR knockout heart homogenates. MLU: mean light unit of 15 measurements. (C) Effects of enzyme inhibitors on the level of O2•− production by wild-type heart homogenates. The results were presented as percentage of the control (without inhibitor). *P<0.05 for indicated values versus the control value in the same figure. _n_=12.
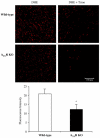
Fig. 2
Dihydroethidium (DHE) fluorescence detection of the ROS production in cardiac sections. Cardiac sections from wild-type and A2AR were incubated with DHE in the presence or absence of tiron (an O2•− scavenger). The DHE fluorescence was visualised under confocal microscopy and quantified. *P<0.05 for A2AR knockout versus wild-type controls. _n_=18 sections from 6 hearts/per group.
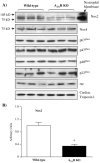
Fig. 3
Immunoblotting for the protein expression of NADPH oxidase subunits in wild-type and A2AR knockout hearts. (A) A neutrophil membrane preparation was used as a positive control for the detection of Nox2. Cardiac troponin I was used as a loading control. (B) Protein bands were quantified densitometrically and normalised to the expression of cardiac troponin I in the same sample. The results were expressed as arbitrary units. *P<0.05 for A2AR knockout versus wild-type controls. _n_=12.
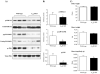
Fig. 4
Differences in cardiac MAPK activation and heart/body weight ratio between wild-type and A2AR knockout mice. (A) Immunoblotting for the expression of total and phosphorylated ERK1/2, p38MAPK, and JNK detected by phospho-specific monoclonal antibodies. (B) Protein bands were quantified densitometrically and the levels of MAPK phosphorylation were normalised to the total protein levels of these molecules in the same samples and expressed as arbitrary units. _n_=12 hearts. (C) Body weights, heart weights, and heart/body weight ratio of wild-type and A2AR knockout mice. _n_=24 mice. *P<0.05 for A2AR knockout versus wild-type controls.
Fig. 5
The effect of SCH58261 on cardiac ROS production. (A) Kinetic measurement of NADPH-dependent O2•− production detected by lucigenin chemiluminescence in cardiac homogenates from wild-type mice treated in vivo with vehicle or SCH58261 (3 mg/kg body weight). Tiron (an O2•− scavenger) and apocynin (an NADPH oxidase inhibitor) were used to confirm the specificity of the assay. (B) Difference in NADPH-dependent O2•− production between heart homogenates from vehicle-treated (0 mg of SCH58261) and SCH58261 (3 and 10 mg/kg body weight)-treated mice. _n_=12 animals/per group. (C) The effect of SCH58261 on ROS production by cultured H9C2 cardiac myocytes. The cell homogenate from 2×106 cells was used for each measurement. _n_=3 independent cell cultures. MLU: mean light unit of 15 measurements. *P<0.05 for indicated values versus control value (0 mg SCH58261).
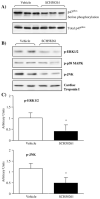
Fig. 6
The effect of SCH58261 treatment on cardiac p47phox phosphorylation and MAPK activation. (A) Top panel: p47phox was immunoprecipitated down and detected by a phospho-serine-specific monoclonal antibody. Total p47phox was detected in parallel as loading controls. (B) ERK1/2, p38MAPK, and JNK phosphorylation detected by phospho-specific monoclonal antibodies. (C) Protein bands were quantified densitometrically and the results were normalised to the total protein levels of these molecules in the same samples and expressed as arbitrary units. _n_=12 hearts. *P<0.05 for indicated value versus vehicle control.
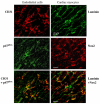
Fig. 7
Confocal microscopy for the cellular expression of p47phox and Nox2 in cardiac sections. Endothelial cells (left panel) were labeled by CD31 (red). Cardiac myocytes (right panel) were outlined by laminin expressed in the sarcolemmal membrane (green). The yellow fluorescence in the lower panels shows the expression of p47phox (green, left middle panel) in endothelial cells, and the expression of Nox2 (red, right middle panel) in cardiac myocytes.
Similar articles
- Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells.
Thakur S, Du J, Hourani S, Ledent C, Li JM. Thakur S, et al. J Biol Chem. 2010 Dec 17;285(51):40104-13. doi: 10.1074/jbc.M110.184606. Epub 2010 Oct 12. J Biol Chem. 2010. PMID: 20940302 Free PMC article. - NOX2 Activation of Natural Killer T Cells Is Blocked by the Adenosine A2A Receptor to Inhibit Lung Ischemia-Reperfusion Injury.
Sharma AK, LaPar DJ, Stone ML, Zhao Y, Mehta CK, Kron IL, Laubach VE. Sharma AK, et al. Am J Respir Crit Care Med. 2016 May 1;193(9):988-99. doi: 10.1164/rccm.201506-1253OC. Am J Respir Crit Care Med. 2016. PMID: 26757359 Free PMC article. - Involvement of NADPH oxidase in A2A adenosine receptor-mediated increase in coronary flow in isolated mouse hearts.
Zhou Z, Rajamani U, Labazi H, Tilley SL, Ledent C, Teng B, Mustafa SJ. Zhou Z, et al. Purinergic Signal. 2015 Jun;11(2):263-73. doi: 10.1007/s11302-015-9451-x. Epub 2015 Apr 25. Purinergic Signal. 2015. PMID: 25911169 Free PMC article. - Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase.
Ushio-Fukai M, Alexander RW. Ushio-Fukai M, et al. Mol Cell Biochem. 2004 Sep;264(1-2):85-97. doi: 10.1023/b:mcbi.0000044378.09409.b5. Mol Cell Biochem. 2004. PMID: 15544038 Review. - Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy.
Ushio-Fukai M, Nakamura Y. Ushio-Fukai M, et al. Cancer Lett. 2008 Jul 18;266(1):37-52. doi: 10.1016/j.canlet.2008.02.044. Epub 2008 Apr 10. Cancer Lett. 2008. PMID: 18406051 Free PMC article. Review.
Cited by
- Adenosine A2A receptor-dependent proliferation of pulmonary endothelial cells is mediated through calcium mobilization, PI3-kinase and ERK1/2 pathways.
Ahmad A, Schaack JB, White CW, Ahmad S. Ahmad A, et al. Biochem Biophys Res Commun. 2013 May 10;434(3):566-71. doi: 10.1016/j.bbrc.2013.03.115. Epub 2013 Apr 10. Biochem Biophys Res Commun. 2013. PMID: 23583199 Free PMC article. - Evidence for the involvement of NADPH oxidase in adenosine receptors-mediated control of coronary flow using A1 and A3 knockout mice.
El-Awady MS, Rajamani U, Teng B, Tilley SL, Mustafa SJ. El-Awady MS, et al. Physiol Rep. 2013 Aug 1;1(3):e00070. doi: 10.1002/phy2.70. Physiol Rep. 2013. PMID: 24159377 Free PMC article. - Mechanism of alcohol-induced oxidative stress and neuronal injury.
Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Haorah J, et al. Free Radic Biol Med. 2008 Dec 1;45(11):1542-50. doi: 10.1016/j.freeradbiomed.2008.08.030. Epub 2008 Sep 17. Free Radic Biol Med. 2008. PMID: 18845238 Free PMC article. - A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A-mediated H2O2 production and KATP opening in isolated mouse hearts.
Zhou X, Teng B, Tilley S, Mustafa SJ. Zhou X, et al. Am J Physiol Heart Circ Physiol. 2013 Dec 1;305(11):H1668-79. doi: 10.1152/ajpheart.00495.2013. Epub 2013 Sep 16. Am J Physiol Heart Circ Physiol. 2013. PMID: 24043252 Free PMC article. - Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells.
Thakur S, Du J, Hourani S, Ledent C, Li JM. Thakur S, et al. J Biol Chem. 2010 Dec 17;285(51):40104-13. doi: 10.1074/jbc.M110.184606. Epub 2010 Oct 12. J Biol Chem. 2010. PMID: 20940302 Free PMC article.
References
- Ashton KJ, Peart JN, Morrison RR, Matherne GP, Blackburn MR, Headrick JP. Genetic modulation of adenosine receptor function and adenosine handling in murine hearts: insights and issues. J. Mol. Cell. Cardiol. 2007;42:693–705. - PubMed
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. - PubMed
- Baxter GF. Role of adenosine in delayed preconditioning of myocardium. Cardiovasc. Res. 2002;55:483–494. - PubMed
- Bedard K, Krause KH. The Nox family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. - PubMed
- Bendall JK, Rinze R, Adlam D, Tatham AL, Joe de Bono T, Channon KM. Endothelial Nox2 overexpresison potentiates vascular oxidative stress and hemodynamic response to angiotensin II: study in endothelial-targeted Nox2 transgenic mice. Circ. Res. 2007;100:1016–1025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous