Transcription-dependent gene looping of the HIV-1 provirus is dictated by recognition of pre-mRNA processing signals - PubMed (original) (raw)
Transcription-dependent gene looping of the HIV-1 provirus is dictated by recognition of pre-mRNA processing signals
Kelly J Perkins et al. Mol Cell. 2008.
Abstract
HIV-1 provirus, either as a chromosomal integrant or as an episomal plasmid in HeLa cells, forms a transcription-dependent gene loop structure between the 5'LTR promoter and 3'LTR poly(A) signal. Flavopiridol-mediated inhibition of RNA polymerase II elongation blocks 5' to 3'LTR juxtaposition, indicating that this structure is maintained during transcription. Analysis of mutant or hybrid HIV-1 plasmids demonstrates that replacement of the 5'LTR promoter with CMV or the 3'LTR poly(A) signal with a synthetic element (SPA) permits gene loop formation, suggesting that these interactions are not retroviral specific. In addition, activation of the 5'LTR poly(A) signal or inactivation of the 3'LTR poly(A) signal abolishes gene loop formation. Overall, we demonstrate that both ongoing transcription and pre-mRNA processing are essential for gene loop formation, and predict that these structures represent a defining feature of active gene transcription.
Figures
Figure 1
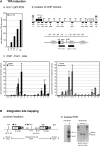
Characterization of Integrated HIV-1 Provirus in U1 Cells (A) (a) HIV-1 mRNA accumulation using qRT-PCR analysis. Values were normalized to 18S rRNA and expressed as a fold induction over untreated U1 cells. (b) Location of ChIP primers relative to the transcription start site (+1) are depicted. Nuc, nucleosomes present on the 5′LTR of latent provirus. Note: P1 does not distinguish between the two LTRs. (c) Pol II qChIP on control (white bars) and TPA-activated U1 cells after 5 (black bars) and 24 hr (gray bars) using the indicated primer and probe sets. Binding was measured relative to B13 (see Experimental Procedures). Error bars represent SEM from n = 3 samples performed in duplicate for each primer set. (B) HIV-1 proviral characterization using iPCR. (a) iPCR primer locations and restriction sites: gray, outer primers; black, nested primers. Open arrows illustrate 3′ HIV-proviral/host cell DNA junctions used to confirm integrate location (see Experimental Procedures). (b) Nested U1 genomic DNA PCR products (+; with Taq polymerase) for integration site mapping and determination of Tat sequences for int-Chr2 (Chr2; Tat1U1) and int-ChrX (ChrX; Tat2U1).
Figure 2
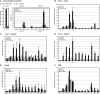
Integrated U1 HIV-1 Proviruses Form Quantitatively Different Looping Conformations (A) Representation of integrated provirus and flanking chromosomal sequence with restriction enzyme sites and primers for BanI and HindIII 3C analysis. Numbers denote distance from 5′ (−) or 3′ (+) proviral ends. Arrows indicate primer direction and name; black/gray arrows refer to primers that detect LTR and the MSD, respectively. HIV-1 long-terminal repeat (LTR) regions (U3, R, and U5), MSD, and polyadenylation sites (pA) are indicated. (B) int-ChrX 3C. Unstimulated (−TPA), cells after 5 hr TPA (+TPA), and control PCR panel (control). Positive lanes (+) signify internal HIV-1 PCR controls on U1 gDNA (control panel) and chromatin (for −/+TPA; see Experimental Procedures). Common PCR primers are shown above the figure, with the second primer shown above each lane. Graphs below represent quantified percentages of 3C product observed compared to PCR control, standardized between − and +TPA samples (using internal PCR controls; see + lane). (C) Quantitative analysis of Tat- or TPA-induced int-ChrX and int-Chr2 loop structures. (a) HIV-1 qRT-PCR at 0, 3, and 5 hr post-TPA treatment with nuc1 primers (Figure 1A) standardized to 18S rRNA transcription. (b) q3C HindIII-digested U1 chromatin analysis; primers used to detect the “loop”: interaction (primers 22/23 for int-Chr2 and X2/X3 for int-ChrX) compared to the adjacent amplified fragment (primers 22/H7 or X2/H7). (D) (a) HIV-1 qRT-PCR treated with 0 (10 μg GFP control), 5, or 10 μg of GFP-tagged Tat protein. (b) q3C HindIII analysis of U1 chromatin treated with 10 μg Tat-GFP or GFP. Error bars represent SEM from n = 6 samples from two separate chromatin preparations (except 22/23 and X2/X3 in Tat induction analysis where n = 9, from three separate chromatin preparations).
Figure 3
p3C Analysis Indicates HIV-1 Loop Formation Occurs on a Plasmid Template (A) (a) RT-PCR analysis of pNL4-3-transfected HeLa cell RNA detecting HIV-1-specific transcripts. HIV, HIV-1 primers; act, actin control primers; M, DNA marker; pUC18, vector backbone, − or + RT. (b) p3C BanI restriction sites and primer position and direction are numbered as in Figure 2A; black and gray arrows denote primers corresponding to LTR and MSD regions, respectively; and plasmid-specific primers are allocated according to the BanI position from the 5′ (−) 3′ (+) proviral ends. (B) BanI p3C analysis shows LTR-LTR and LTR/MSD juxtaposition. Positive PCR panels (control) were used to demonstrate that primer sets amplify correct products. + lane signifies internal HIV-1 PCR controls; common PCR primers are shown above the figure and second primers above each lane. Significant 3C products (∗) and those derived from adjacent fragment ligation (X) are denoted. Graphs below represent quantified percentage of 3C product observed compared to PCR control, as per Figure 2B (note: chromatin preparations for p2 (d) and p4 (e) were concentrated as outlined in the Experimental Procedures). (C) 3C and p3C summary. HIV-1 provirus with restriction sites present in pNL4-3, int-ChrX, int-Chr2, and their flanking regions are as indicated. Major LTR-LTR interactions are shown in black; regions showing partial interaction are in gray. Lower brackets define minimal interacting regions from combined 3C and p3C data.
Figure 4
Occupancy of Pol II and Associated Factors across Transcriptionally Active Provirus and Flanking Chromosomal Regions (A) Asymmetric distribution of Pol II Ser5P (a) Ser2P (b), CDK9 (c), and USF (d) association was determined by qChIP assay in control (white bars) and 5 hr TPA-activated U1 cells (black bars). Immunoprecipitated DNA was analyzed by real-time PCR using the primer sets as described in Figure 1. ChIP signal levels are not comparable between antibodies. Note: P1 does not distinguish between the two LTRs. (B) Flanking chromosomal analysis of proviral integrates indicates differential response to TPA stimulation for (a) int-ChrX and (b) int-Chr2. Error bars represent SEM from n = 3 samples performed in duplicate for each primer set.
Figure 5
Flavopiridol Inactivates HIV-1 Transcription by Blocking CTD Ser2P and Ser5p, Leading to Loss of Gene Loop Structure (A) (a) HIV-1 mRNA levels (qRT-PCR) and (b) q3C (see Figure 2) prior to (white bar) or after 16 hr TPA treatment (black bar) followed by 5 hr of flavopiridol treatment (500 nM; gray bar). (B–F) qChIP analysis for total RNAP II, Ser5P, Ser2P, CDK9, and USF respectively was performed for control (white bars), transcriptionally active (TPA induced; black bars), and transcriptionally blocked (flavopiridol treatment after TPA induction) U1 cells (gray bars). ChIP signal levels are not comparable between antibodies. P1 primers amplified from both 5′ and 3′LTR DNA. Error bars represent SEM from n = 3 samples performed in duplicate for each primer set, except primers 22/23 and X2/X3 (n = 12), which are from four separate chromatin preparations.
Figure 6
Functional MSD and 3′LTR Poly(A) Signals Are Required for Transcription-Dependent Loop Formation of pNL4 HIV-1 Provirus (A) (a) pNL4 5′ end mutant constructs used in p3C analysis showing primers used to detect HIV-1 LTR or CMV promoter (p3) and the major splice donor (MSD; B1). Primer numbering and positions, poly(A), LTR, and MSD regions are as in Figure 3. Arrows indicate transcription start sites. (b) Real-time q-p3C analysis of comparative loop formation using primer p3 (5′LTR) using pNL4, derivatives pNL4-luc, and pNL4.msd and (c) pNL4-luc compared to the CMV promoter construct pNL4-luc.CMV. (B) (a) pNL4-luc 3′ end poly(A) and SPA constructs used in p3C analysis; primer numbering and positions are as in Figure 3, with luciferase (luc+) reporter gene, poly(A), and LTR regions as shown. Real-time q-p3C analysis of comparative loop formation using primer p3 (5′LTR) in analysis of the SPA wild-type and mutant (b) HIV-1 poly(A) and DSE mutants (c). Asterisks denote absence of the B4 primer binding site for SPA constructs. For all graphs, dotted lines show values of pNL4-luc obtained for 5′LTR/MSD and 5′LTR/3′ end (3′LTR) products with (+) or without (−) cotransfected Tat expression vector. Primer p3 was used in combination with the primers depicted below all graphs. Error bars represent SEM between values from separate chromatin preparations performed in triplicate (n = 6), except for p3/B1 (n = 12) and p3/p2 (n = 15).
Figure 7
Ribbon Diagrams Illustrating HIV-1 Interactions as Determined by 3C Analysis (A) Potential interactions in the HIV-1 proviral sequence upon TPA or Tat stimulation for int-ChrX. Also illustrated is the hypothesized conformation of pNL4-3 in (B) wild-type (C) in the absence of a functional 3′ end poly(A) signal (as evidenced by the HIV-1 poly[A] and SPA mutants) and (D) without a functional MSD. Dashed lines represent transcription (with gray and white circles denoting promoter/poly[A] sites respectively), with LTRs, MSD, and poly(A) hexamer as indicated.
Similar articles
- Mutation of the major 5' splice site renders a CMV-driven HIV-1 proviral clone Tat-dependent: connections between transcription and splicing.
Bohne J, Kräusslich HG. Bohne J, et al. FEBS Lett. 2004 Apr 9;563(1-3):113-8. doi: 10.1016/S0014-5793(04)00277-7. FEBS Lett. 2004. PMID: 15063733 - Tat functions to stimulate the elongation properties of transcription complexes paused by the duplicated TAR RNA element of human immunodeficiency virus 2.
García-Martínez LF, Mavankal G, Peters P, Wu-Baer F, Gaynor RB. García-Martínez LF, et al. J Mol Biol. 1995 Dec 1;254(3):350-63. doi: 10.1006/jmbi.1995.0622. J Mol Biol. 1995. PMID: 7490754 - Analysis of a novel defective HTLV-I provirus and detection of a new HTLV-I-induced cellular transcript.
Kubota S, Furuta RA, Siomi H, Maki M, Hatanaka M. Kubota S, et al. FEBS Lett. 1995 Nov 13;375(1-2):31-6. doi: 10.1016/0014-5793(95)01166-c. FEBS Lett. 1995. PMID: 7498474 - HIV Tat, its TARgets and the control of viral gene expression.
Brigati C, Giacca M, Noonan DM, Albini A. Brigati C, et al. FEMS Microbiol Lett. 2003 Mar 14;220(1):57-65. doi: 10.1016/S0378-1097(03)00067-3. FEMS Microbiol Lett. 2003. PMID: 12644228 Review. - Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator.
Marcello A, Zoppé M, Giacca M. Marcello A, et al. IUBMB Life. 2001 Mar;51(3):175-81. doi: 10.1080/152165401753544241. IUBMB Life. 2001. PMID: 11547919 Review.
Cited by
- Metazoan promoters: emerging characteristics and insights into transcriptional regulation.
Lenhard B, Sandelin A, Carninci P. Lenhard B, et al. Nat Rev Genet. 2012 Mar 6;13(4):233-45. doi: 10.1038/nrg3163. Nat Rev Genet. 2012. PMID: 22392219 Review. - Gene loops enhance transcriptional directionality.
Tan-Wong SM, Zaugg JB, Camblong J, Xu Z, Zhang DW, Mischo HE, Ansari AZ, Luscombe NM, Steinmetz LM, Proudfoot NJ. Tan-Wong SM, et al. Science. 2012 Nov 2;338(6107):671-5. doi: 10.1126/science.1224350. Epub 2012 Sep 27. Science. 2012. PMID: 23019609 Free PMC article. - Detection of gene loops by 3C in yeast.
Singh BN, Ansari A, Hampsey M. Singh BN, et al. Methods. 2009 Aug;48(4):361-7. doi: 10.1016/j.ymeth.2009.02.018. Epub 2009 Mar 6. Methods. 2009. PMID: 19269325 Free PMC article. - Transcription termination by nuclear RNA polymerases.
Richard P, Manley JL. Richard P, et al. Genes Dev. 2009 Jun 1;23(11):1247-69. doi: 10.1101/gad.1792809. Genes Dev. 2009. PMID: 19487567 Free PMC article. - Anticancer Alkaloids from Trees: Development into Drugs.
Isah T. Isah T. Pharmacogn Rev. 2016 Jul-Dec;10(20):90-99. doi: 10.4103/0973-7847.194047. Pharmacogn Rev. 2016. PMID: 28082790 Free PMC article. Review.
References
- Ashe M.P., Griffin P., James W., Proudfoot N.J. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 1995;9:3008–3025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources